Deregulated inflammasome signaling in disease
- PMID: 21884175
- PMCID: PMC3170132
- DOI: 10.1111/j.1600-065X.2011.01042.x
Deregulated inflammasome signaling in disease
Abstract
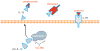
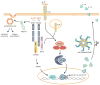
Inflammasomes are multi-protein complexes that sense microbial molecules and endogenous danger signals in intracellular compartments. Inflammasome assembly results in caspase-1 activation, which in turn drives maturation and secretion of the pro-inflammatory cytokines interleukin 1β (IL-1β) and IL-18, and induces pyroptosis to eliminate the infectious agent. The importance of inflammasomes in regulating immune responses was recognized with the discovery of polymorphisms in genes encoding inflammasome components and their linkage to aberrant production of IL-1β and IL-18 in autoimmune and hereditary periodic fevers syndromes. We review the current knowledge on the role of inflammasomes in regulating innate and adaptive immune responses with an emphasis on the role of these immune complexes in autoinflammatory disorders and autoimmune diseases such as colitis, type I diabetes, multiple sclerosis and vitiligo.
© 2011 John Wiley & Sons A/S.
Conflict of interest statement
The author declares no competing financial interests.
Figures
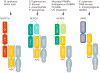
References
-
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. - PubMed
-
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. - PubMed
-
- Call ME, Wucherpfennig KW. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol. 2005;23:101–125. - PubMed
-
- Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

