Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts
- PMID: 21884525
- PMCID: PMC3221000
- DOI: 10.1111/j.1471-4159.2011.07467.x
Ethanol causes the redistribution of L1 cell adhesion molecule in lipid rafts
Abstract
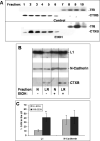
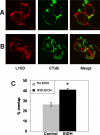
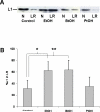
Fetal alcohol spectrum disorder is estimated to affect 1% of live births. The similarities between children with fetal alcohol syndrome and those with mutations in the gene encoding L1 cell adhesion molecule (L1) implicates L1 as a target of ethanol developmental neurotoxicity. Ethanol specifically inhibits the neurite outgrowth promoting function of L1 at pharmacologic concentrations. Emerging evidence shows that localized disruption of the lipid rafts reduces L1-mediated neurite outgrowth. We hypothesize that ethanol impairment of the association of L1 with lipid rafts is a mechanism underlying ethanol's inhibition of L1-mediated neurite outgrowth. In this study, we examine the effects of ethanol on the association of L1 and lipid rafts. We show that, in vitro, L1 but not N-cadherin shifts into lipid rafts following treatment with 25 mM ethanol. The ethanol concentrations causing this effect are similar to those inhibiting L1-mediated neurite outgrowth. Increasing chain length of the alcohol demonstrates the same cutoff as that previously shown for inhibition of L1-L1 binding. In addition, in cerebellar granule neurons in which lipid rafts are disrupted with methyl-beta-cyclodextrin, the rate of L1-mediated neurite outgrowth on L1-Fc is reduced to background rate and that this background rate is not ethanol sensitive. These data indicate that ethanol may inhibit L1-mediated neurite outgrowth by retarding L1 trafficking through a lipid raft compartment.
© 2011 The Authors. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Figures







Similar articles
-
Effect of lipid raft disruption on ethanol inhibition of l1 adhesion.Alcohol Clin Exp Res. 2014 Nov;38(11):2707-11. doi: 10.1111/acer.12556. Alcohol Clin Exp Res. 2014. PMID: 25421507 Free PMC article.
-
Choline partially prevents the impact of ethanol on the lipid raft dependent functions of l1 cell adhesion molecule.Alcohol Clin Exp Res. 2014 Nov;38(11):2722-30. doi: 10.1111/acer.12554. Alcohol Clin Exp Res. 2014. PMID: 25421509 Free PMC article.
-
Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells.J Biol Chem. 1999 May 7;274(19):13264-70. doi: 10.1074/jbc.274.19.13264. J Biol Chem. 1999. PMID: 10224086 Free PMC article.
-
Ethanol inhibits L1 cell adhesion molecule activation of mitogen-activated protein kinases.J Neurochem. 2006 Mar;96(5):1480-90. doi: 10.1111/j.1471-4159.2006.03649.x. J Neurochem. 2006. PMID: 16478533 Free PMC article.
-
L1 cell adhesion molecule signal cascades: targets for ethanol developmental neurotoxicity.Neurotoxicology. 2001 Oct;22(5):625-33. doi: 10.1016/s0161-813x(01)00034-1. Neurotoxicology. 2001. PMID: 11770884 Review.
Cited by
-
Choline supplementation prevents the effects of bilirubin on cerebellar-mediated behavior in choline-restricted Gunn rat pups.Pediatr Res. 2021 May;89(6):1414-1419. doi: 10.1038/s41390-020-01187-7. Epub 2020 Oct 7. Pediatr Res. 2021. PMID: 33027804 Free PMC article.
-
Cholesterol in Brain Development and Perinatal Brain Injury: More than a Building Block.Curr Neuropharmacol. 2022;20(7):1400-1412. doi: 10.2174/1570159X19666211111122311. Curr Neuropharmacol. 2022. PMID: 34766894 Free PMC article. Review.
-
Issues in assessing the health risks of n-butanol.J Appl Toxicol. 2020 Jan;40(1):72-86. doi: 10.1002/jat.3820. Epub 2019 Jun 24. J Appl Toxicol. 2020. PMID: 31231852 Free PMC article. Review.
-
Bilirubin inhibits lipid raft dependent functions of L1 cell adhesion molecule in rat pup cerebellar granule neurons.Pediatr Res. 2021 May;89(6):1389-1395. doi: 10.1038/s41390-020-01156-0. Epub 2020 Sep 16. Pediatr Res. 2021. PMID: 32937649 Free PMC article.
-
L1 cell adhesion molecule signaling is inhibited by ethanol in vivo.Alcohol Clin Exp Res. 2013 Mar;37(3):383-9. doi: 10.1111/j.1530-0277.2012.01944.x. Epub 2012 Oct 10. Alcohol Clin Exp Res. 2013. PMID: 23050935 Free PMC article.
References
-
- Carpenter-Hyland EP, Chandler LJ. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur. J. Neurosci. 2006;24(12):3496–506. - PubMed
-
- Charness M, Safran R, Perides G. Ethanol inhibits neural cell-cell adhesion. J. Biol. Chem. 1994;269:9304–9309. - PubMed
-
- Dai Q, Zhang J, Pruett SB. Ethanol alters cellular activation and CD14 partitioning in lipid rafts. Biochem. Biophys. Res. Commun. 2005;332(1):37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

