Integrated safety in tocilizumab clinical trials
- PMID: 21884601
- PMCID: PMC3308069
- DOI: 10.1186/ar3455
Integrated safety in tocilizumab clinical trials
Abstract
Introduction: The efficacy and safety of tocilizumab in patients with rheumatoid arthritis have been evaluated in a comprehensive phase 3 program. Patients from these randomized trials could receive tocilizumab treatment in open-label extension trials. Here, the long-term safety profile of tocilizumab, using pooled data from all of these trials, is reported.
Methods: Cumulative safety data (as of February 6, 2009) from five core phase 3 trials, two ongoing extension trials, and one clinical pharmacology study were analyzed. Two patient populations were evaluated: an all-control population (n = 4,199), which included all patients randomly assigned in the placebo-controlled portions of the five core studies, and an all-exposed population (n = 4,009), which included patients from any of the eight studies who received at least one dose of tocilizumab.
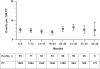
Results: Total exposure to tocilizumab was 8,580 patient years (PY), and total duration of observation was 9,414 PY. Overall adverse event (AE) and serious AE (SAE) rates were 278.2/100 PY and 14.4/100 PY, respectively. These events included serious infections (4.7/100 PY), opportunistic infections (0.23/100 PY), gastrointestinal perforations (0.28/100 PY), malignancy (1.1/100 PY), myocardial infarction (0.25/100 PY), and stroke (0.19/100 PY). The rates of SAEs and serious infections were stable over time; no increase with prolonged exposure was noted.
Conclusions: The longer-term safety profile of tocilizumab (mean treatment duration, 2.4 years) is consistent with that observed in the phase 3 studies (duration up to 1 year).
Figures

References
-
- Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R. OPTION Investigators. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. - DOI - PubMed
-
- Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumor necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. - PMC - PubMed
-
- Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T, Gomez-Reino JJ. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. - DOI - PubMed
-
- Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland A-M, Vernon E, Ambs P, Fleischmann R. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63:609–621. doi: 10.1002/art.30158. - DOI - PubMed
-
- Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, Siri DA, Tomsic M, Alecock E, Woodworth T, Genovese MC. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

