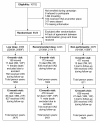
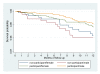
The impact of different doses of vitamin A supplementation on male and female mortality. A randomised trial from Guinea-Bissau
- PMID: 21884606
- PMCID: PMC3175170
- DOI: 10.1186/1471-2431-11-77
The impact of different doses of vitamin A supplementation on male and female mortality. A randomised trial from Guinea-Bissau
Abstract
Background: Vitamin A supplementation (VAS) given to children between 6 months and 5 years of age is known to reduce mortality in low-income countries. We have previously observed that girls benefit more from a lower dose of VAS than the one recommended by WHO, the effect being strongest if diphtheria-tetanus-pertussis vaccine (DTP) was the most recent vaccination. We aimed to test these observations.
Methods: During national immunisations days in Guinea-Bissau, West Africa, combining oral polio vaccination and VAS, we randomised 8626 children between 6 months and 5 years of age to receive the dose of VAS recommended by WHO or half this dose. Mortality rate ratios (MRRs) were assessed after 6 and 12 month.
Results: The overall mortality rate among participants was lower than expected. There was no significant difference in mortality at 6 months and 12 months of follow up between the low dose VAS group and the recommended dose VAS group. The MRRs were 1.23 (0.60-2.54) after 6 months and 1.17 (0.73-1.87) after 12 months. This tendency was similar in boys and girls. The low dose was not associated with lower mortality in girls if the most recent vaccine was DTP (MRR = 0.60 (0.14-2.50) after 6 months).
Conclusion: Our sample size does not permit firm conclusions since mortality was lower than expected. We could not confirm a beneficial effect of a lower dose of VAS on mortality in girls.
Trial registration: The study was registered under clinicaltrials.gov, number NCT00168636.
Figures
References
-
- Beaton GH, Martorell R, McCabe G, L'abbé KA, Edmonston B, Ross AC. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. University of Toronto. 1993.
-
- Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev. 2010. p. CD008524. - PubMed
-
- WHO/UNISEF/IVAGG Task Force. Vitamin A supplement. A guide to their use in the treatment and prevention of vitamin A deficiency and xerophthalmia. Geneva, WHO. 1997.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical