Aggrecan, an unusual polyelectrolyte: review of solution behavior and physiological implications
- PMID: 21884828
- PMCID: PMC3226867
- DOI: 10.1016/j.actbio.2011.08.011
Aggrecan, an unusual polyelectrolyte: review of solution behavior and physiological implications
Abstract
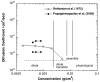
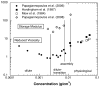
Aggrecan is a high-molecular-weight, bottlebrush-shaped, negatively charged biopolymer that forms supermolecular complexes with hyaluronic acid. In the extracellular matrix of cartilage, aggrecan-hyaluronic acid complexes are interspersed in a collagen meshwork and provide the osmotic properties required to resist deswelling under compressive load. In this review we compile aggrecan solution behavior from different experimental techniques, and discuss them in the context of concentration regimes that were identified in osmotic pressure experiments. At low concentrations, aggrecan exhibits microgel-like behavior. With increasing concentration, the bottlebrushes self-assemble into large complexes. In the physiological concentration range (2<c(aggrecan)<8% w/w), the physical properties of the solution are dominated by repulsive electrostatic interactions between aggrecan complexes. We discuss the consequences of the bottlebrush architecture on the polyelectrolyte characteristics of the aggrecan molecule, and its implications for cartilage properties and function.
Published by Elsevier Ltd.
Figures





References
-
- Ng L, Grodzinsky AJ, Patwari P, Sandy J, Plaas A, Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. Journal of Structural Biology. 2003;143:242–57. - PubMed
-
- Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12:19–32. - PubMed
-
- Iozzo R. Proteoglycans: Structure, Biology, and Molecular Interactions. New York: Marcel Dekker Inc; 2000.
-
- Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58:255–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

