A biotin switch-based proteomics approach identifies 14-3-3ζ as a target of Sirt1 in the metabolic regulation of caspase-2
- PMID: 21884983
- PMCID: PMC3417139
- DOI: 10.1016/j.molcel.2011.07.028
A biotin switch-based proteomics approach identifies 14-3-3ζ as a target of Sirt1 in the metabolic regulation of caspase-2
Abstract
While lysine acetylation in the nucleus is well characterized, comparatively little is known about its significance in cytoplasmic signaling. Here we show that inhibition of the Sirt1 deacetylase, which is primarily cytoplasmic in cancer cell lines, sensitizes these cells to caspase-2-dependent death. To identify relevant Sirt1 substrates, we developed a proteomics strategy, enabling the identification of a range of putative substrates, including 14-3-3ζ, a known direct regulator of caspase-2. We show here that inhibition of Sirtuin activity accelerates caspase activation and overrides caspase-2 suppression by nutrient abundance. Furthermore, 14-3-3ζ is acetylated prior to caspase activation, and supplementation of Xenopus egg extract with glucose-6-phosphate, which promotes caspase-2/14-3-3ζ binding, enhances 14-3-3ζ-directed Sirtuin activity. Conversely, inhibiting Sirtuin activity promotes14-3-3ζ dissociation from caspase-2 in both egg extract and human cultured cells. These data reveal a role for Sirt1 in modulating apoptotic sensitivity, in response to metabolic changes, by antagonizing 14-3-3ζ acetylation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

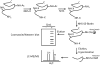

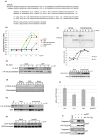
References
-
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. - PubMed
-
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

