Parainfluenza virus 5-based vaccine vectors expressing vaccinia virus (VACV) antigens provide long-term protection in mice from lethal intranasal VACV challenge
- PMID: 21885079
- PMCID: PMC3177979
- DOI: 10.1016/j.virol.2011.08.005
Parainfluenza virus 5-based vaccine vectors expressing vaccinia virus (VACV) antigens provide long-term protection in mice from lethal intranasal VACV challenge
Abstract
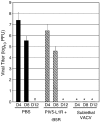
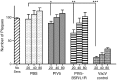
To test the potential for parainfluenza virus 5 (PIV5)-based vectors to provide protection from vaccinia virus (VACV) infection, PIV5 was engineered to express secreted VACV L1R and B5R proteins, two important antigens for neutralization of intracellular mature (IMV) and extracellular enveloped (EEV) virions, respectively. Protection of mice from lethal intranasal VACV challenge required intranasal immunization with PIV5-L1R/B5R in a prime-boost protocol, and correlated with low VACV-induced pathology in the respiratory tract and anti-VACV neutralizing antibody. Mice immunized with PIV5-L1R/B5R showed some disease symptoms following VACV challenge such as loss of weight and hunching, but these symptoms were delayed and less severe than with unimmunized control mice. While immunization with PIV5 expressing B5R alone conferred at least some protection, the most effective immunization included the PIV5 vector expressing L1R alone or in combination with PIV5-B5R. PIV5-L1R/B5R vectors elicited protection from VACV challenge even when CD8+ cells were depleted, but not in the case of mice that were defective in B cell production. Mice were protected from VACV challenge out to at least 1.5 years after immunization with PIV5-L1R/B5R vectors, and showed significant levels of anti-VACV neutralizing antibodies. These results demonstrate the potential for PIV5-based vectors to provide long lasting protection against complex human respiratory pathogens such as VACV, but also highlight the need to understand mechanisms for the generation of strong immune responses against poorly immunogenic viral proteins.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures






References
-
- Aldaz-Carroll L., Whitbeck J.C., Ponce de Leon M., Lou H., Pannell L.K., Lebowitz J., Fogg C., White C.L., Moss B., Eisenberg R.J., Cohen G.H. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. 2005;341:59–71. - PubMed
-
- Bell E., Shamim M., Whitbeck J.C., Sfyroera G., Lambris J.D., Isaacs S.N. Antibodies against the EEV B5R protein are mainly responsible for EEV neutralization capacity of vaccinia virus immune globulin. Virology. 2004;325:425–431. - PubMed
-
- Belyakov I.M., Earl P., Dzutsev A., Kuznetsov V.A., Lemon M., Wyatt L.S., Snyder J.T., Ahlers J.D., Franchini G., Moss B., Berzofsky J.A. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. U.S.A. 2005;100:9458–9463. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

