Molecular organization of Drosophila neuroendocrine cells by Dimmed
- PMID: 21885285
- PMCID: PMC3184372
- DOI: 10.1016/j.cub.2011.08.015
Molecular organization of Drosophila neuroendocrine cells by Dimmed
Abstract
Background: In Drosophila, the basic-helix-loop-helix protein DIMM coordinates the molecular and cellular properties of all major neuroendocrine cells, irrespective of the secretory peptides they produce. When expressed by nonneuroendocrine neurons, DIMM confers the major properties of the regulated secretory pathway and converts such cells away from fast neurotransmission and toward a neuroendocrine state.
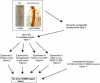
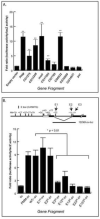
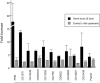
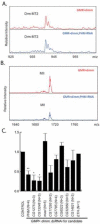
Results: We first identified 134 transcripts upregulated by DIMM in embryos and then evaluated them systematically using diverse assays (including embryo in situ hybridization, in vivo chromatin immunoprecipitation, and cell-based transactivation assays). We conclude that of eleven strong candidates, six are strongly and directly controlled by DIMM in vivo. The six targets include several large dense-core vesicle (LDCV) proteins, but also proteins in non-LDCV compartments such as the RNA-associated protein Maelstrom. In addition, a functional in vivo assay, combining transgenic RNA interference with MS-based peptidomics, revealed that three DIMM targets are especially critical for its action. These include two well-established LDCV proteins, the amidation enzyme PHM and the ascorbate-regenerating electron transporter cytochrome b(561-1). The third key DIMM target, CAT-4 (CG13248), has not previously been associated with peptide neurosecretion-it encodes a putative cationic amino acid transporter, closely related to the Slimfast arginine transporter. Finally, we compared transcripts upregulated by DIMM with those normally enriched in DIMM neurons of the adult brain and found an intersection of 18 DIMM-regulated genes, which included all six direct DIMM targets.
Conclusions: The results provide a rigorous molecular framework with which to describe the fundamental regulatory organization of diverse neuroendocrine cells.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr. Rev. 1999;20:3–21. - PubMed
-
- Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiol. 2006;21:124–133. - PubMed
-
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol. Rev. 2001;81:1197–1267. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- P30 NS057105/NS/NINDS NIH HHS/United States
- P30 DA018310/DA/NIDA NIH HHS/United States
- NS036570/NS/NINDS NIH HHS/United States
- P01 NS044232/NS/NINDS NIH HHS/United States
- P30-NS057105/NS/NINDS NIH HHS/United States
- EY016807/EY/NEI NIH HHS/United States
- P30 NS045713/NS/NINDS NIH HHS/United States
- NS031609/NS/NINDS NIH HHS/United States
- R01 NS036570/NS/NINDS NIH HHS/United States
- P01-NS044232/NS/NINDS NIH HHS/United States
- P30-NS045713/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- NS21749/NS/NINDS NIH HHS/United States
- R01 NS021749/NS/NINDS NIH HHS/United States
- R01 NS031609/NS/NINDS NIH HHS/United States
- P30-DA018310/DA/NIDA NIH HHS/United States
- R01 EY016807/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

