Vitamin C degradation products and pathways in the human lens
- PMID: 21885436
- PMCID: PMC3199460
- DOI: 10.1074/jbc.M111.245100
Vitamin C degradation products and pathways in the human lens
Abstract
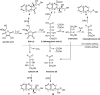
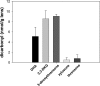
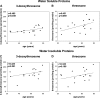
Vitamin C and its degradation products participate in chemical modifications of proteins in vivo through non-enzymatic glycation (Maillard reaction) and formation of different products called advanced glycation end products. Vitamin C levels are particularly high in selected tissues, such as lens, brain and adrenal gland, and its degradation products can inflict substantial protein damage via formation of advanced glycation end products. However, the pathways of in vivo vitamin C degradation are poorly understood. Here we have determined the levels of vitamin C oxidation and degradation products dehydroascorbic acid, 2,3-diketogulonic acid, 3-deoxythreosone, xylosone, and threosone in the human lens using o-phenylenediamine to trap both free and protein-bound adducts. In the protein-free fraction and water-soluble proteins (WSP), all five listed degradation products were identified. Dehydroascorbic acid, 2,3-diketogulonic acid, and 3-deoxythreosone were the major products in the protein-free fraction, whereas in the WSP, 3-deoxythreosone was the most abundant measured dicarbonyl. In addition, 3-deoxythreosone in WSP showed positive linear correlation with age (p < 0.05). In water-insoluble proteins, only 3-deoxythreosone and threosone were detected, whereby the level of 3-deoxythreosone was ∼20 times higher than the level of threosone. The identification of 3-deoxythreosone as the major degradation product bound to human lens proteins provides in vivo evidence for the non-oxidative pathway of dehydroascorbate degradation into erythrulose as a major pathway for vitamin C degradation in vivo.
Figures


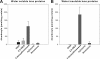

References
-
- Varma S. D., Richards R. D. (1988) Ophthalmic Res. 20, 164–173 - PubMed
-
- Cheng R., Feng Q., Ortwerth B. J. (2006) Biochim. Biophys. Acta 1762, 533–543 - PubMed
-
- Argirov O. K., Lin B., Ortwerth B. J. (2004) J. Biol. Chem. 279, 6487–6495 - PubMed
-
- Atalay A., Ogus A., Bateman O., Slingsby C. (1998) Biochimie 80, 283–288 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

