Lentivirus-mediated expression of cDNA and shRNA slows degeneration in retinitis pigmentosa
- PMID: 21885480
- PMCID: PMC4405537
- DOI: 10.1258/ebm.2011.011053
Lentivirus-mediated expression of cDNA and shRNA slows degeneration in retinitis pigmentosa
Abstract
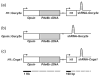
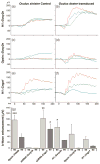
Mutations in Pde6b lead to high levels of signaling molecules cyclic guanosine monophosphate (cGMP) and Ca(2+), which ultimately result in photoreceptor cell death in certain forms of retinitis pigmentosa (RP). The level of cGMP, which is controlled by opposing activities of guanylate cyclase (GUCY) and photoreceptor phosphodiesterase-6 (PDE6), regulates the opening of cyclic nucleotide-gated ion channels [CNG] and thereby controls Ca(2+) influx into the outer segments. Using a lentiviral gene therapy approach, we have previously shown that degeneration can be temporarily slowed either by introducing wild-type PDE6β or knocking down expression of GUCY2E and CNGA1 in photoreceptors of Pde6b(H620Q), a mouse model for RP. Rescue was transient with either approach. Therefore, we tested a novel combination therapy using bipartite lentiviral vectors designed to both introduce wild-type PDE6β expression and knockdown GUCY2E or CNGA1. Immunoblot analysis shows simultaneous increases in PDE6β and decreases in GUCY2E or CNGA1 in retinas transduced by the vectors, indicating successful transduction. In Pde6b(H620Q) mutants, we observe rescue of photoreceptor function and an increase in photoreceptor rows as compared with untreated controls. However, no evidence of prolonged rescue beyond the limit of the previously tested single therapy was observed.
Figures


References
-
- Bird AC. Retinal photoreceptor dystrophies: the LI. Edward Jackson Memorial Lecture. Am J Ophthalmol. 1995;119:543–62. - PubMed
-
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. - PubMed
-
- Davis RJ, Tosi J, Janisch KM, Kasanuki JM, Wang NK, Kong J, Tsui I, Cilluffo M, Woodruff ML, Fain GL, Lin CS, Tsang SH. Functional rescue of degenerating photoreceptors in mice homozygous for a hypomorphic cGMP phosphodiesterase 6 allele (Pde6bH620Q) Invest Ophthalmol Vis Sci. 2008;49:5067–76. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

