Molecular mechanism of scanning and start codon selection in eukaryotes
- PMID: 21885680
- PMCID: PMC3165540
- DOI: 10.1128/MMBR.00008-11
Molecular mechanism of scanning and start codon selection in eukaryotes
Abstract
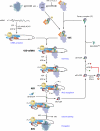
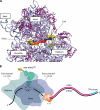
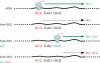
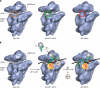
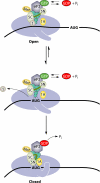
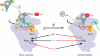
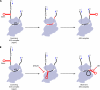
The correct translation of mRNA depends critically on the ability to initiate at the right AUG codon. For most mRNAs in eukaryotic cells, this is accomplished by the scanning mechanism, wherein the small (40S) ribosomal subunit attaches to the 5' end of the mRNA and then inspects the leader base by base for an AUG in a suitable context, using complementarity with the anticodon of methionyl initiator tRNA (Met-tRNAiMet) as the key means of identifying AUG. Over the past decade, a combination of yeast genetics, biochemical analysis in reconstituted systems, and structural biology has enabled great progress in deciphering the mechanism of ribosomal scanning. A robust molecular model now exists, describing the roles of initiation factors, notably eukaryotic initiation factor 1 (eIF1) and eIF1A, in stabilizing an "open" conformation of the 40S subunit with Met-tRNAiMet bound in a low-affinity state conducive to scanning and in triggering rearrangement into a "closed" conformation incompatible with scanning, which features Met-tRNAiMet more tightly bound to the "P" site and base paired with AUG. It has also emerged that multiple DEAD-box RNA helicases participate in producing a single-stranded "landing pad" for the 40S subunit and in removing the secondary structure to enable the mRNA to traverse the 40S mRNA-binding channel in the single-stranded form for base-by-base inspection in the P site.
Figures






References
-
- Acker M. G., Shin B. S., Dever T. E., Lorsch J. R. 2006. Interaction between eukaryotic initiation factors 1A and 5B is required for efficient ribosomal subunit joining. J. Biol. Chem. 281:8469–8475 - PubMed
-
- Algire M. A., Maag D., Lorsch J. R. 2005. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 20:251–262 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

