The universally conserved prokaryotic GTPases
- PMID: 21885683
- PMCID: PMC3165542
- DOI: 10.1128/MMBR.00009-11
The universally conserved prokaryotic GTPases
Abstract
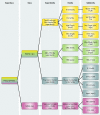
Members of the large superclass of P-loop GTPases share a core domain with a conserved three-dimensional structure. In eukaryotes, these proteins are implicated in various crucial cellular processes, including translation, membrane trafficking, cell cycle progression, and membrane signaling. As targets of mutation and toxins, GTPases are involved in the pathogenesis of cancer and infectious diseases. In prokaryotes also, it is hard to overestimate the importance of GTPases in cell physiology. Numerous papers have shed new light on the role of bacterial GTPases in cell cycle regulation, ribosome assembly, the stress response, and other cellular processes. Moreover, bacterial GTPases have been identified as high-potential drug targets. A key paper published over 2 decades ago stated that, "It may never again be possible to capture [GTPases] in a family portrait" (H. R. Bourne, D. A. Sanders, and F. McCormick, Nature 348:125-132, 1990) and indeed, the last 20 years have seen a tremendous increase in publications on the subject. Sequence analysis identified 13 bacterial GTPases that are conserved in at least 75% of all bacterial species. We here provide an overview of these 13 protein subfamilies, covering their cellular functions as well as cellular localization and expression levels, three-dimensional structures, biochemical properties, and gene organization. Conserved roles in eukaryotic homologs will be discussed as well. A comprehensive overview summarizing current knowledge on prokaryotic GTPases will aid in further elucidating the function of these important proteins.
Figures








References
-
- Akiyama T., et al. 2001. Mammalian homologue of E. coli Ras-like GTPase (ERA) is a possible apoptosis regulator with RNA binding activity. Genes Cells 6:987–1001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

