A gustotopic map of taste qualities in the mammalian brain
- PMID: 21885776
- PMCID: PMC3523322
- DOI: 10.1126/science.1204076
A gustotopic map of taste qualities in the mammalian brain
Abstract
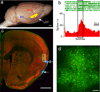
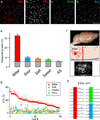
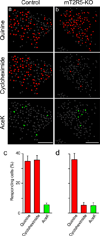
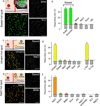
The taste system is one of our fundamental senses, responsible for detecting and responding to sweet, bitter, umami, salty, and sour stimuli. In the tongue, the five basic tastes are mediated by separate classes of taste receptor cells each finely tuned to a single taste quality. We explored the logic of taste coding in the brain by examining how sweet, bitter, umami, and salty qualities are represented in the primary taste cortex of mice. We used in vivo two-photon calcium imaging to demonstrate topographic segregation in the functional architecture of the gustatory cortex. Each taste quality is represented in its own separate cortical field, revealing the existence of a gustotopic map in the brain. These results expose the basic logic for the central representation of taste.
Figures
Comment in
-
Neuroscience. Sweet here, salty there: evidence for a taste map in the mammalian brain.Science. 2011 Sep 2;333(6047):1213. doi: 10.1126/science.333.6047.1213. Science. 2011. PMID: 21885750 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

