Chemical and genetic engineering of selective ion channel-ligand interactions
- PMID: 21885782
- PMCID: PMC3210548
- DOI: 10.1126/science.1206606
Chemical and genetic engineering of selective ion channel-ligand interactions
Abstract
Ionic flux mediates essential physiological and behavioral functions in defined cell populations. Cell type-specific activators of diverse ionic conductances are needed for probing these effects. We combined chemistry and protein engineering to enable the systematic creation of a toolbox of ligand-gated ion channels (LGICs) with orthogonal pharmacologic selectivity and divergent functional properties. The LGICs and their small-molecule effectors were able to activate a range of ionic conductances in genetically specified cell types. LGICs constructed for neuronal perturbation could be used to selectively manipulate neuron activity in mammalian brains in vivo. The diversity of ion channel tools accessible from this approach will be useful for examining the relationship between neuronal activity and animal behavior, as well as for cell biological and physiological applications requiring chemical control of ion conductance.
Figures

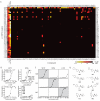
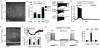

Comment in
-
Molecular matchmaking for neural control.Nat Methods. 2011 Nov;8(11):898. doi: 10.1038/nmeth.1759. Nat Methods. 2011. PMID: 22148157 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

