Estrogen signaling and cardiovascular disease
- PMID: 21885836
- PMCID: PMC3398381
- DOI: 10.1161/CIRCRESAHA.110.236687
Estrogen signaling and cardiovascular disease
Abstract
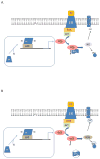
Estrogen has pleiotropic effects on the cardiovascular system. The mechanisms by which estrogen confers these pleiotropic effects are undergoing active investigation. Until a decade ago, all estrogen signaling was thought to occur by estrogen binding to nuclear estrogen receptors (estrogen receptor-α and estrogen receptor-β), which bind to DNA and function as ligand-activated transcription factors. Estrogen binding to the receptor alters gene expression, thereby altering cell function. Estrogen also binds to nuclear estrogen receptors that are tethered to the plasma membrane, resulting in acute activation of signaling kinases such as PI3K. An orphan G-protein-coupled receptor, G-protein-coupled receptor 30, can also bind estrogen and activate acute signaling pathways. Thus, estrogen can alter cell function by binding to different estrogen receptors. This article reviews the different estrogen receptors and their signaling mechanisms, discusses mechanisms that regulate estrogen receptor levels and locations, and considers the cardiovascular effects of estrogen signaling.
Figures


References
-
- McInerney EM, Katzenellenbogen BS. Different regions in activation function-1 of the human estrogen receptor required for antiestrogen- and estradiol-dependent transcription activation. J Biol Chem. 1996;271(39):24172–24178. - PubMed
-
- Schwend T, Gustafsson JA. False positives in MALDI-TOF detection of ERbeta in mitochondria. Biochem Biophys Res Commun. 2006;343(3):707–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

