VMY-1-103 is a novel CDK inhibitor that disrupts chromosome organization and delays metaphase progression in medulloblastoma cells
- PMID: 21885916
- PMCID: PMC3367670
- DOI: 10.4161/cbt.12.9.17682
VMY-1-103 is a novel CDK inhibitor that disrupts chromosome organization and delays metaphase progression in medulloblastoma cells
Abstract
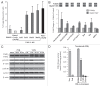
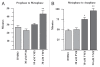
Medulloblastoma is the most prevalent of childhood brain malignancies, constituting 25% of childhood brain tumors. Craniospinal radiotherapy is a standard of care, followed by a 12mo regimen of multi-agent chemotherapy. For children less than 3 y of age, irradiation is avoided due to its destructive effects on the developing nervous system. Long-term prognosis is worst for these youngest children and more effective treatment strategies with a better therapeutic index are needed. VMY-1-103, a novel dansylated analog of purvalanol B, was previously shown to inhibit cell cycle progression and proliferation in prostate and breast cancer cells more effectively than purvalanol B. In the current study, we have identified new mechanisms of action by which VMY-1-103 affected cellular proliferation in medulloblastoma cells. VMY-1-103, but not purvalanol B, significantly decreased the proportion of cells in S phase and increased the proportion of cells in G(2)/M. VMY-1-103 increased the sub G(1) fraction of apoptotic cells, induced PARP and caspase-3 cleavage and increased the levels of the Death Receptors DR4 and DR5, Bax and Bad while decreasing the number of viable cells, all supporting apoptosis as a mechanism of cell death. p21(CIP1/WAF1) levels were greatly suppressed. Importantly, we found that while both VMY and flavopiridol inhibited intracellular CDK1 catalytic activity, VMY-1-103 was unique in its ability to severely disrupt the mitotic spindle apparatus significantly delaying metaphase and disrupting mitosis. Our data suggest that VMY-1-103 possesses unique antiproliferative capabilities and that this compound may form the basis of a new candidate drug to treat medulloblastoma.
Figures

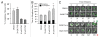


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
