Evidence that CED-9/Bcl2 and CED-4/Apaf-1 localization is not consistent with the current model for C. elegans apoptosis induction
- PMID: 21886181
- PMCID: PMC3278724
- DOI: 10.1038/cdd.2011.104
Evidence that CED-9/Bcl2 and CED-4/Apaf-1 localization is not consistent with the current model for C. elegans apoptosis induction
Abstract
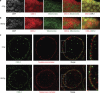
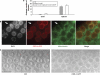
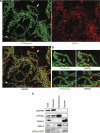
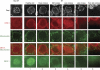
In C. elegans, the BH3-only domain protein EGL-1, the Apaf-1 homolog CED-4 and the CED-3 caspase are required for apoptosis induction, whereas the Bcl-2 homolog CED-9 prevents apoptosis. Mammalian B-cell lymphoma 2 (Bcl-2) inhibits apoptosis by preventing the release of the Apaf-1 (apoptotic protease-activating factor 1) activator cytochrome c from mitochondria. In contrast, C. elegans CED-9 is thought to inhibit CED-4 by sequestering it at the outer mitochondrial membrane by direct binding. We show that CED-9 associates with the outer mitochondrial membrane within distinct foci that do not overlap with CED-4, which is predominantly perinuclear and does not localize to mitochondria. CED-4 further accumulates in the perinuclear space in response to proapoptotic stimuli such as ionizing radiation. This increased accumulation depends on EGL-1 and is abrogated in ced-9 gain-of-function mutants. CED-4 accumulation is not sufficient to trigger apoptosis execution, even though it may prime cells for apoptosis. Our results suggest that the cell death protection conferred by CED-9 cannot be solely explained by a direct interaction with CED-4.
Figures



Similar articles
-
The pro-apoptotic function of the C. elegans BCL-2 homolog CED-9 requires interaction with the APAF-1 homolog CED-4.Sci Adv. 2024 Oct 11;10(41):eadn0325. doi: 10.1126/sciadv.adn0325. Epub 2024 Oct 9. Sci Adv. 2024. PMID: 39383227 Free PMC article.
-
Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans.Science. 2008 Oct 3;322(5898):110-5. doi: 10.1126/science.1158111. Science. 2008. PMID: 18832646 Free PMC article.
-
A molecular switch that governs mitochondrial fusion and fission mediated by the BCL2-like protein CED-9 of Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2011 Oct 11;108(41):E813-22. doi: 10.1073/pnas.1103218108. Epub 2011 Sep 23. Proc Natl Acad Sci U S A. 2011. PMID: 21949250 Free PMC article.
-
Noncanonical cell death programs in the nematode Caenorhabditis elegans.Cell Death Differ. 2008 Jul;15(7):1124-31. doi: 10.1038/cdd.2008.56. Epub 2008 Apr 25. Cell Death Differ. 2008. PMID: 18437162 Review.
-
2:1 Stoichiometry of the CED-4-CED-9 complex and the tetrameric CED-4: insights into the regulation of CED-3 activation.Cell Cycle. 2006 Jan;5(1):31-4. doi: 10.4161/cc.5.1.2263. Epub 2006 Jan 18. Cell Cycle. 2006. PMID: 16294007 Review.
Cited by
-
Identification of a novel ADAMTS9/GON-1 function for protein transport from the ER to the Golgi.Mol Biol Cell. 2012 May;23(9):1728-41. doi: 10.1091/mbc.E11-10-0857. Epub 2012 Mar 14. Mol Biol Cell. 2012. PMID: 22419820 Free PMC article.
-
Fine-tuning of chromatin composition and Polycomb recruitment by two Mi2 homologues during C. elegans early embryonic development.Epigenetics Chromatin. 2016 Sep 15;9:39. doi: 10.1186/s13072-016-0091-3. eCollection 2016. Epigenetics Chromatin. 2016. PMID: 27651832 Free PMC article.
-
LIN-3/EGF promotes the programmed cell death of specific cells in Caenorhabditis elegans by transcriptional activation of the pro-apoptotic gene egl-1.PLoS Genet. 2014 Aug 21;10(8):e1004513. doi: 10.1371/journal.pgen.1004513. eCollection 2014 Aug. PLoS Genet. 2014. PMID: 25144461 Free PMC article.
-
Tryptophanyl-tRNA synthetase-1 (WARS-1) depletion and high tryptophan concentration lead to genomic instability in Caenorhabditis elegans.Cell Death Discov. 2024 Apr 4;10(1):165. doi: 10.1038/s41420-024-01917-4. Cell Death Discov. 2024. PMID: 38575580 Free PMC article.
-
RNA-binding protein GLD-1/quaking genetically interacts with the mir-35 and the let-7 miRNA pathways in Caenorhabditis elegans.Open Biol. 2013 Nov 20;3(11):130151. doi: 10.1098/rsob.130151. Open Biol. 2013. PMID: 24258276 Free PMC article.
References
-
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. - PubMed
-
- Conradt B, Horvitz HR. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. - PubMed
-
- Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356:494–499. - PubMed
-
- del Peso L, Gonzalez VM, Inohara N, Ellis RE, Nunez G. Disruption of the CED-9.CED-4 complex by EGL-1 is a critical step for programmed cell death in Caenorhabditis elegans. J Biol Chem. 2000;275:27205–27211. - PubMed
-
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

