Linking neural activity and molecular oscillations in the SCN
- PMID: 21886186
- PMCID: PMC4356239
- DOI: 10.1038/nrn3086
Linking neural activity and molecular oscillations in the SCN
Abstract
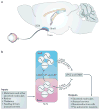
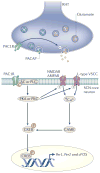
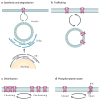
Neurons in the suprachiasmatic nucleus (SCN) function as part of a central timing circuit that drives daily changes in our behaviour and underlying physiology. A hallmark feature of SCN neuronal populations is that they are mostly electrically silent during the night, start to fire action potentials near dawn and then continue to generate action potentials with a slow and steady pace all day long. Sets of currents are responsible for this daily rhythm, with the strongest evidence for persistent Na(+) currents, L-type Ca(2+) currents, hyperpolarization-activated currents (I(H)), large-conductance Ca(2+) activated K(+) (BK) currents and fast delayed rectifier (FDR) K(+) currents. These rhythms in electrical activity are crucial for the function of the circadian timing system, including the expression of clock genes, and decline with ageing and disease. This article reviews our current understanding of the ionic and molecular mechanisms that drive the rhythmic firing patterns in the SCN.
Conflict of interest statement
The author declares no competing financial interests.
Figures
References
-
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. - PubMed
-
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. - PubMed
-
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. - PubMed
-
- Herzog ED. Neurons and networks in daily rhythms. Nature Rev Neurosci. 2007;8:790–802. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

