Dynamic niches in the origination and differentiation of haematopoietic stem cells
- PMID: 21886187
- PMCID: PMC4040463
- DOI: 10.1038/nrm3184
Dynamic niches in the origination and differentiation of haematopoietic stem cells
Erratum in
- Nat Rev Mol Cell Biol. 2011;13(1):12
Abstract
Haematopoietic stem cells (HSCs) are multipotent, self-renewing progenitors that generate all mature blood cells. HSC function is tightly controlled to maintain haematopoietic homeostasis, and this regulation relies on specialized cells and factors that constitute the haematopoietic 'niche', or microenvironment. Recent discoveries, aided in part by technological advances in in vivo imaging, have engendered a new appreciation for the dynamic nature of the niche, identifying novel cellular and acellular niche components and uncovering fluctuations in the relative importance of these components over time. These new insights significantly improve our understanding of haematopoiesis and raise fundamental questions about what truly constitutes a stem cell niche.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
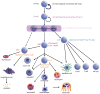


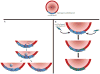
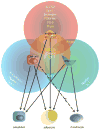
References
-
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. This seminal paper was first to apply the concept of the niche to stem cell biology, postulating that loss of HSC association with the niche would result in differentiation. - PubMed
-
- Eliasson P, Jonsson J. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. J Cell Physiol. 2009;222:17–22. - PubMed
-
- Eliasson P, et al. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term-reconstituting hematopoietic stem cells during in vitro culture. Experimental Hematology. 2010;38:301–310.e2. - PubMed
-
- Kulkeaw K, Ishitani T, Kanemaru T, Fucharoen S, Sugiyama D. Cold exposure down-regulates zebrafish hematopoiesis. Biochemical and Biophysical Research Communications. 2010;394:859–864. - PubMed
-
- Adamo L, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–5. Demonstrates that shear stress increases Runx1 expression and colony-forming potential in embryonic stem cells differentiated in vitro into HSCs, and in hematopoietic precursors in the AGM region of mouse embryos. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

