Monoclonal antibodies recognizing the non-tandem repeat regions of the human mucin MUC4 in pancreatic cancer
- PMID: 21886786
- PMCID: PMC3160300
- DOI: 10.1371/journal.pone.0023344
Monoclonal antibodies recognizing the non-tandem repeat regions of the human mucin MUC4 in pancreatic cancer
Abstract
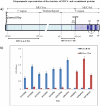
The MUC4 mucin is a high molecular weight, membrane-bound, and highly glycosylated protein. It is a multi-domain protein that is putatively cleaved into a large mucin-like subunit (MUC4α) and a C-terminal growth-factor like subunit (MUC4β). MUC4 plays critical roles in physiological and pathological conditions and is aberrantly overexpressed in several cancers, including those of the pancreas, cervix, breast and lung. It is also a potential biomarker for the diagnosis, prognosis and progression of several malignancies. Further, MUC4 plays diverse functional roles in cancer initiation and progression as evident from its involvement in oncogenic transformation, proliferation, inhibition of apoptosis, motility and invasion, and resistance to chemotherapy in human cancer cells. We have previously generated a monoclonal antibody 8G7, which is directed against the TR region of MUC4, and has been extensively used to study the expression of MUC4 in several malignancies. Here, we describe the generation of anti-MUC4 antibodies directed against the non-TR regions of MUC4. Recombinant glutathione-S-transferase (GST)-fused MUC4α fragments, both upstream (MUC4α-N-Ter) and downstream (MUC4α-C-Ter) of the TR domain, were used as immunogens to immunize BALB/c mice. Following cell fusion, hybridomas were screened using the aforementioned recombinant proteins ad lysates from human pancreatic cell lines. Three anti MUC4α-N-Ter and one anti-MUC4α-C-Ter antibodies were characterized by several inmmunoassays including enzyme-linked immunosorbent assay (ELISA), immunoblotting, immunofluorescene, flow cytometry and immunoprecipitation using MUC4 expressing human pancreatic cancer cell lines. The antibodies also reacted with the MUC4 in human pancreatic tumor sections in immunohistochemical analysis. The new domain-specific anti-MUC4 antibodies will serve as important reagents to study the structure-function relationship of MUC4 domains and for the development of MUC4-based diagnostics and therapeutics.
Conflict of interest statement
Figures
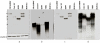




References
-
- Sheng Z, Wu K, Carraway KL, Fregien N. Molecular cloning of the transmembrane component of the 13762 mammary adenocarcinoma sialomucin complex. A new member of the epidermal growth factor superfamily. J Biol Chem. 1992;267:16341–16346. - PubMed
-
- Wu K, Fregien N, Carraway KL. Molecular cloning and sequencing of the mucin subunit of a heterodimeric, bifunctional cell surface glycoprotein complex of ascites rat mammary adenocarcinoma cells. J Biol Chem. 1994;269:11950–11955. - PubMed
-
- Copin MC, Devisme L, Buisine MP, Marquette CH, Wurtz A, et al. From normal respiratory mucosa to epidermoid carcinoma: expression of human mucin genes. Int J Cancer. 2000;86:162–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

