Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation
- PMID: 21886795
- PMCID: PMC3158765
- DOI: 10.1371/journal.pone.0023530
Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation
Abstract
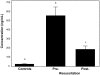
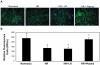
The early use of fresh frozen plasma as a resuscitative agent after hemorrhagic shock has been associated with improved survival, but the mechanism of protection is unknown. Hemorrhagic shock causes endothelial cell dysfunction and we hypothesized that fresh frozen plasma would restore endothelial integrity and reduce syndecan-1 shedding after hemorrhagic shock. A prospective, observational study in severely injured patients in hemorrhagic shock demonstrated significantly elevated levels of syndecan-1 (554±93 ng/ml) after injury, which decreased with resuscitation (187±36 ng/ml) but was elevated compared to normal donors (27±1 ng/ml). Three pro-inflammatory cytokines, interferon-γ, fractalkine, and interleukin-1β, negatively correlated while one anti-inflammatory cytokine, IL-10, positively correlated with shed syndecan-1. These cytokines all play an important role in maintaining endothelial integrity. An in vitro model of endothelial injury then specifically examined endothelial permeability after treatment with fresh frozen plasma orlactated Ringers. Shock or endothelial injury disrupted junctional integrity and increased permeability, which was improved with fresh frozen plasma, but not lactated Ringers. Changes in endothelial cell permeability correlated with syndecan-1 shedding. These data suggest that plasma based resuscitation preserved endothelial syndecan-1 and maintained endothelial integrity, and may help to explain the protective effects of fresh frozen plasma after hemorrhagic shock.
Conflict of interest statement
Figures

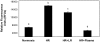



References
-
- Sauaia A, Moore FA, Moore EE, Moore EE, Moser KS, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. - PubMed
-
- Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. - PubMed
-
- Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, et al. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA multicenter trial. J Am Coll Surg. 2009;208:1–13. - PubMed
-
- Borgman M, Spinella PC, Perkins JG, Grathwohl KW, Repine T, et al. Blood product replacement affects survival in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

