The targeting of plasmalemmal ceramide to mitochondria during apoptosis
- PMID: 21886813
- PMCID: PMC3158777
- DOI: 10.1371/journal.pone.0023706
The targeting of plasmalemmal ceramide to mitochondria during apoptosis
Abstract
Ceramide is a key lipid mediator of cellular processes such as differentiation, proliferation, growth arrest and apoptosis. During apoptosis, ceramide is produced within the plasma membrane. Although recent data suggest that the generation of intracellular ceramide increases mitochondrial permeability, the source of mitochondrial ceramide remains unknown. Here, we determine whether a stress-mediated plasmalemmal pool of ceramide might become available to the mitochondria of apoptotic cells. We have previously established annexin A1--a member of a family of Ca(2+) and membrane-binding proteins--to be a marker of ceramide platforms. Using fluorescently tagged annexin A1, we show that, upon its generation within the plasma membrane, ceramide self-associates into platforms that subsequently invaginate and fuse with mitochondria. An accumulation of ceramide within the mitochondria of apoptotic cells was also confirmed using a ceramide-specific antibody. Electron microscopic tomography confirmed that upon the formation of ceramide platforms, the invaginated regions of the plasma membrane extend deep into the cytoplasm forming direct physical contacts with mitochondrial outer membranes. Ceramide might thus be directly transferred from the plasma membrane to the mitochondrial outer membrane. It is conceivable that this "kiss-of-death" increases the permeability of the mitochondrial outer membrane thereby triggering apoptosis.
Conflict of interest statement
Figures



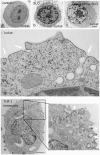
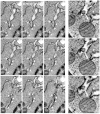
References
-
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. - PubMed
-
- Martinez-Abundis E, Correa F, Pavon N, Zazueta C. Bax distribution into mitochondrial detergent-resistant microdomains is related to ceramide and cholesterol content in postischemic hearts. FEBS J. 2009;276:5579–5588. - PubMed
-
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

