Development and validation of amisulpride in human plasma by HPLC coupled with tandem mass spectrometry and its application to a pharmacokinetic study
- PMID: 21886905
- PMCID: PMC3163372
- DOI: 10.3797/scipharm.1105-12
Development and validation of amisulpride in human plasma by HPLC coupled with tandem mass spectrometry and its application to a pharmacokinetic study
Abstract
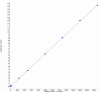
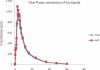
In this study, authors developed a simple, sensitive and specific liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for quantification of Amisulpride in human plasma using Amisulpride-d(5) as an internal standard (IS). Chromatographic separation was performed on Zorbax Bonus-RP C18, 4.6 × 75 mm, 3.5 μm column with an isocratic mobile phase composed of 0.2% formic acid:methanol (35:65 v/v), at a flow-rate of 0.5 mL/min. Amisulpride, Amisulpride-d(5) was detected at m/z 370.1→242.1 and 375.1→242.1. The drug and the IS were extracted by a liquid-liquid extraction method. The method was validated over a linear concentration range of 2.0-2500.0 ng/mL for Amisulpride with a correlation coefficient of (r(2)) ≥ 0.9982. This method demonstrated intra- and inter-day precision within 0.9 to 1.7 and 1.5 to 2.8 % and intra- and inter-day accuracy within 98.3 to 101.5 and 96.0 to 101.0 % for Amisulpride. Amisulpride was found to be stable at 3 freeze-thaw cycles, bench top and auto sampler stability studies. The developed method was successfully applied to a pharmacokinetic study.
Keywords: Amisulpride; Liquid chromatography–tandam mass spectrometry; Method Validation; Method development.
Figures
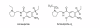




References
-
- Coukell AJ, Spencer CM, Benfield P. Amisulpride: A Review of its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Efficacy in the Management of Schizophrenia. CNS Drugs. 1996;6:237–243. doi: 10.2165/00023210-199606030-00006. - DOI
-
- Costa e Silva JA. The public health impact of anxiety disorders: a WHO perspective. Acta Psychiatr Scand Suppl. 1998;393:2–5. - PubMed
-
- Xiberas X, Martinot JL, Mallet L, Artiges E, Canal M, Loc’h C, Mazière B, Paillère-Martinot ML. In Vivo Extrastriatal and Striatal D2 Dopamine Receptor Blockade by Amisulpride in Schizophrenia. J Clin Psychopharmacol. 2001;21:207–214. - PubMed
LinkOut - more resources
Full Text Sources
