Development of Microfabricated Magnetic Actuators for Removing Cellular Occlusion
- PMID: 21886945
- PMCID: PMC3163296
- DOI: 10.1088/0960-1317/21/5/054006
Development of Microfabricated Magnetic Actuators for Removing Cellular Occlusion
Abstract
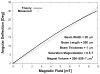
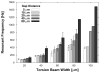
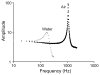
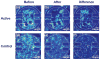
Here we report on the development of torsional magnetic microactuators for displacing biological materials in implantable catheters. Static and dynamic behaviors of the devices were characterized in air and in fluid using optical experimental methods. The devices were capable of achieving large deflections (>60°) and had resonant frequencies that ranged from 70 Hz to 1.5 kHz in fluid. The effect of long-term actuation (>2.5 · 10(8) cycles) was quantified using resonant shift as the metric (Δf < 2%). Cell-clearing capabilities of the devices were evaluated by examining the effect of actuation on a layer of aggressively growing adherent cells. On average, actuated microdevices removed 37.4% of the adherent cell layer grown over the actuator surface. The effect of actuation time, deflection angle, and beam geometry were evaluated. The experimental results indicate that physical removal of adherent cells at the microscale is feasible using magnetic microactuation.
Figures
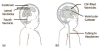












References
-
- Spina Bifida and Hydrocephalus Association of Nova Scotia. Hydrocephalus Information Page.
-
- Pollack I, Albright A, Adelson P. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Neurosurgery. 1999;45:1399–1411. - PubMed
-
- Kestle J, et al. Long-term follow-up date from the shunt design trial. Pediatric Neurosurgery. 2000;33:230–236. - PubMed
-
- Choux M, Di Rocco C, Hockley AD, Walker ML, editors. Pediatric Neurosurgery. Churchill Livingstone; 1999.
-
- National Institute of Neurological Disorders and Stroke. NINDS Hydrocephalus Information Page.
Grants and funding
LinkOut - more resources
Full Text Sources
