Relaxin-3-deficient mice showed slight alteration in anxiety-related behavior
- PMID: 21887138
- PMCID: PMC3156976
- DOI: 10.3389/fnbeh.2011.00050
Relaxin-3-deficient mice showed slight alteration in anxiety-related behavior
Abstract
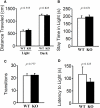
Relaxin-3 is a neuropeptide belonging to the relaxin/insulin superfamily. Studies using rodents have revealed that relaxin-3 is predominantly expressed in neurons in the nucleus incertus (NI) of the pons, the axons of which project to forebrain regions including the hypothalamus. There is evidence that relaxin-3 is involved in several functions, including food intake and stress responses. In the present study, we generated relaxin-3 gene knockout (KO) mice and examined them using a range of behavioral tests of sensory/motor functions and emotion-related behaviors. The results revealed that relaxin-3 KO mice exhibited normal growth and appearance, and were generally indistinguishable from wild genotype littermates. There was no difference in bodyweight among genotypes until at least 28 weeks after birth. In addition, there were no significant differences between wild-type and KO mice in locomotor activity, social interaction, hot plate test performance, fear conditioning, depression-like behavior, and Y-maze test performance. However, in the elevated plus maze test, KO mice exhibited a robust increase in the tendency to enter open arms, although they exhibited normal performance in a light/dark transition test and showed no difference from wild-type mice in the time spent in central area in the open field test. On the other hand, a significant increase in the acoustic startle response was observed in KO mice. These results indicate that relaxin-3 is slightly involved in the anxiety-related behavior.
Keywords: anxiety; elevated plus maze; gene knockout; mouse; relaxin-3.
Figures










References
-
- Anseloni V. Z., Motta V., Lima G., Brandao M. L. (1995). Behavioral and pharmacological validation of the elevated plus maze constructed with transparent walls. Braz. J. Med. Biol. Res. 28, 597–601 - PubMed
-
- Bathgate R. A., Samuel C. S., Burazin T. C., Layfield S., Claasz A. A., Reytomas I. G., Dawson N. F., Zhao C., Bond C., Summers R. J., Parry L. J., Wade J. D., Tregear G. W. (2002). Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. Novel members of the relaxin peptide family. J. Biol. Chem. 277, 1148–115710.1074/jbc.M107882200 - DOI - PubMed
-
- Bullesbach E. E., Yang S., Schwabe C. (1992). The receptor-binding site of human relaxin II. A dual prong-binding mechanism. J. Biol. Chem. 267, 22957–22960 - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

