BPR1K653, a novel Aurora kinase inhibitor, exhibits potent anti-proliferative activity in MDR1 (P-gp170)-mediated multidrug-resistant cancer cells
- PMID: 21887256
- PMCID: PMC3160846
- DOI: 10.1371/journal.pone.0023485
BPR1K653, a novel Aurora kinase inhibitor, exhibits potent anti-proliferative activity in MDR1 (P-gp170)-mediated multidrug-resistant cancer cells
Abstract
Background: Over-expression of Aurora kinases promotes the tumorigenesis of cells. The aim of this study was to determine the preclinical profile of a novel pan-Aurora kinase inhibitor, BPR1K653, as a candidate for anti-cancer therapy. Since expression of the drug efflux pump, MDR1, reduces the effectiveness of various chemotherapeutic compounds in human cancers, this study also aimed to determine whether the potency of BPR1K653 could be affected by the expression of MDR1 in cancer cells.
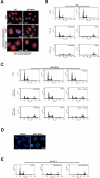
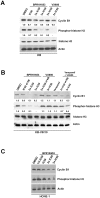
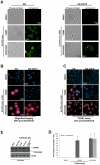
Principal findings: BPR1K653 specifically inhibited the activity of Aurora-A and Aurora-B kinase at low nano-molar concentrations in vitro. Anti-proliferative activity of BPR1K653 was evaluated in various human cancer cell lines. Results of the clonogenic assay showed that BPR1K653 was potent in targeting a variety of cancer cell lines regardless of the tissue origin, p53 status, or expression of MDR1. At the cellular level, BPR1K653 induced endo-replication and subsequent apoptosis in both MDR1-negative and MDR1-positive cancer cells. Importantly, it showed potent activity against the growth of xenograft tumors of the human cervical carcinoma KB and KB-derived MDR1-positive KB-VIN10 cells in nude mice. Finally, BPR1K653 also exhibited favorable pharmacokinetic properties in rats.
Conclusions and significance: BPR1K653 is a novel potent anti-cancer compound, and its potency is not affected by the expression of the multiple drug resistant protein, MDR1, in cancer cells. Therefore, BPR1K653 is a promising anti-cancer compound that has potential for the management of various malignancies, particularly for patients with MDR1-related drug resistance after prolonged chemotherapeutic treatments.
Conflict of interest statement
Figures



References
-
- Cheung CH, Coumar MS, Hsieh HP, Chang JY. Aurora kinase inhibitors in preclinical and clinical testing. Expert Opin Investig Drugs. 2009;18:379–398. - PubMed
-
- Cheung CH, Coumar MS, Chang JY, Hsieh HP. Aurora kinase inhibitor patents and agents in clinical testing: an update (2009–10) This article is an update to aurora kinase inhibitors review, which appeared in: Expert Opin. Ther. Patents 2009, 19, 1–36 and Expert Opin. Investig. Drugs 2009, 18, 1–20. Expert Opin Ther Pat. 2010;21:857–884. - PubMed
-
- Vischioni B, Oudejans JJ, Vos W, Rodriguez JA, Giaccone G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol Cancer Ther. 2006;5:2905–2913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

