Age-related immunity to meningococcal serogroup C vaccination: an increase in the persistence of IgG2 correlates with a decrease in the avidity of IgG
- PMID: 21887261
- PMCID: PMC3160848
- DOI: 10.1371/journal.pone.0023497
Age-related immunity to meningococcal serogroup C vaccination: an increase in the persistence of IgG2 correlates with a decrease in the avidity of IgG
Abstract
Background: All children and adolescents between 1 and 19 years of age in The Netherlands received a single meningococcal serogroup C conjugate (MenCC) vaccine in 2002. During follow-up 4-5 years later, the persistence of MenC polysaccharide-specific IgG was found to be dependent on age of vaccination with higher IgG levels in the oldest immunized age categories.
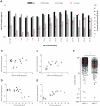
Methods and findings: Two cross-sectional population-based serum banks, collected in 1995/1996 and in 2006/2007, were used for this study. We measured MenC polysaccharide-specific IgM, the IgG1 and IgG2 subclasses and determined the avidity of the IgG antibodies. We report that the age-related persistence of IgG after immunization with the MenCC vaccine seemed to result from an increase of IgG2 levels with age, while IgG1 levels remained stable throughout the different age-cohorts. Furthermore, an age-related increase in IgM levels was observed, correlating with the persistence of IgG antibodies with age. It is noteworthy that the increase in IgG2 correlated with a reduced IgG-avidity with age.
Conclusion: These date indicate that the classical characteristics of a T-cell-dependent antibody response as elicited by protein based vaccines might not be completely applicable when conjugate vaccines are administered to older children and adolescents up to 18 years of age. The response elicited by the MenCC vaccine seemed to be more a mixture of both T cell dependent and T cell independent responses in terms of humoral immunological characteristics.
Conflict of interest statement
Figures
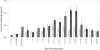
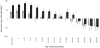
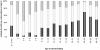
References
-
- Ada G, Isaacs D. Carbohydrate-protein conjugate vaccines. Clin Microbiol Infect. 2003;9:79–85. - PubMed
-
- De Greeff SC, de Melker HE, Spanjaard L, Schouls LM, van Derende A. Protection from routine vaccination at the age of 14 months with meningococcal serogroup C conjugate vaccine in the Netherlands. Pediatr Infect Dis J. 2006;25:79–80. - PubMed
-
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–20. - PubMed
-
- Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl 1):S58–S67. - PubMed
-
- Cano R, Larrauri A, Mateo S, Alcala B, Salcedo C, et al. Impact of the meningococcal C conjugate vaccine in Spain: an epidemiological and microbiological decision. Euro Surveill. 2004;9:11–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

