Isolation and characterization of CvIV4: a pain inducing α-scorpion toxin
- PMID: 21887265
- PMCID: PMC3160894
- DOI: 10.1371/journal.pone.0023520
Isolation and characterization of CvIV4: a pain inducing α-scorpion toxin
Abstract
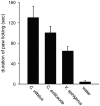
Background: Among scorpion species, the Buthidae produce the most deadly and painful venoms. However, little is known regarding the venom components that cause pain and their mechanism of action. Using a paw-licking assay (Mus musculus), this study compared the pain-inducing capabilities of venoms from two species of New World scorpion (Centruroides vittatus, C. exilicauda) belonging to the neurotoxin-producing family Buthidae with one species of non-neurotoxin producing scorpion (Vaejovis spinigerus) in the family Vaejovidae. A pain-inducing α-toxin (CvIV4) was isolated from the venom of C. vittatus and tested on five Na(+) channel isoforms.
Principal findings: C. vittatus and C. exilicauda venoms produced significantly more paw licking in Mus than V. spinigerus venom. CvIV4 produced paw licking in Mus equivalent to the effects of whole venom. CvIV4 slowed the fast inactivation of Na(v)1.7, a Na(+) channel expressed in peripheral pain-pathway neurons (nociceptors), but did not affect the Na(v)1.8-based sodium currents of these neurons. CvIV4 also slowed the fast inactivation of Na(v)1.2, Na(v)1.3 and Na(v)1.4. The effects of CvIV4 are similar to Old World α-toxins that target Na(v)1.7 (AahII, BmK MI, LqhIII, OD1), however the primary structure of CvIV4 is not similar to these toxins. Mutant Na(v)1.7 channels (D1586A and E1589Q, DIV S3-S4 linker) reduced but did not abolish the effects of CvIV4.
Conclusions: This study: 1) agrees with anecdotal evidence suggesting that buthid venom is significantly more painful than non-neurotoxic venom; 2) demonstrates that New World buthids inflict painful stings via toxins that modulate Na(+) channels expressed in nociceptors; 3) reveals that Old and New World buthids employ similar mechanisms to produce pain. Old and New World α-toxins that target Na(v)1.7 have diverged in sequence, but the activity of these toxins is similar. Pain-inducing toxins may have evolved in a common ancestor. Alternatively, these toxins may be the product of convergent evolution.
Conflict of interest statement
Figures




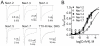
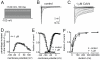



Similar articles
-
Isolation and characterization of two novel scorpion toxins: The alpha-toxin-like CeII8, specific for Na(v)1.7 channels and the classical anti-mammalian CeII9, specific for Na(v)1.4 channels.Toxicon. 2010 Sep 15;56(4):613-23. doi: 10.1016/j.toxicon.2010.06.008. Epub 2010 Jun 19. Toxicon. 2010. PMID: 20600228
-
Negative-shift activation, current reduction and resurgent currents induced by β-toxins from Centruroides scorpions in sodium channels.Toxicon. 2012 Feb;59(2):283-93. doi: 10.1016/j.toxicon.2011.12.003. Epub 2011 Dec 16. Toxicon. 2012. PMID: 22200496
-
Evolutionary diversification of Mesobuthus α-scorpion toxins affecting sodium channels.Mol Cell Proteomics. 2012 Jan;11(1):M111.012054. doi: 10.1074/mcp.M111.012054. Epub 2011 Oct 3. Mol Cell Proteomics. 2012. PMID: 21969612 Free PMC article.
-
An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch.Toxicon. 2002 Sep;40(9):1239-58. doi: 10.1016/s0041-0101(02)00142-3. Toxicon. 2002. PMID: 12220709 Review.
-
Autonomic effects of some scorpion venoms and toxins.Clin Exp Pharmacol Physiol. 2002 Sep;29(9):795-801. doi: 10.1046/j.1440-1681.2002.03726.x. Clin Exp Pharmacol Physiol. 2002. PMID: 12165045 Review.
Cited by
-
Scorpion Peptides and Ion Channels: An Insightful Review of Mechanisms and Drug Development.Toxins (Basel). 2023 Mar 24;15(4):238. doi: 10.3390/toxins15040238. Toxins (Basel). 2023. PMID: 37104176 Free PMC article. Review.
-
Pain-Causing Venom Peptides: Insights into Sensory Neuron Pharmacology.Toxins (Basel). 2017 Dec 27;10(1):15. doi: 10.3390/toxins10010015. Toxins (Basel). 2017. PMID: 29280959 Free PMC article. Review.
-
The Specific Effects of OD-1, a Peptide Activator, on Voltage-Gated Sodium Current and Seizure Susceptibility.Int J Mol Sci. 2020 Nov 4;21(21):8254. doi: 10.3390/ijms21218254. Int J Mol Sci. 2020. PMID: 33158049 Free PMC article.
-
Scorpion Toxin, BmP01, Induces Pain by Targeting TRPV1 Channel.Toxins (Basel). 2015 Sep 14;7(9):3671-87. doi: 10.3390/toxins7093671. Toxins (Basel). 2015. PMID: 26389953 Free PMC article.
-
Deciphering Scorpion Toxin-Induced Pain: Molecular Mechanisms and Ion Channel Dynamics.Int J Biol Sci. 2025 Apr 21;21(7):2921-2934. doi: 10.7150/ijbs.109713. eCollection 2025. Int J Biol Sci. 2025. PMID: 40384871 Free PMC article. Review.
References
-
- Fet V, Lowe G. Family Buthidae. In: Fet V, Sissom WD, Lowe G, Braunwalder ME, editors. Catalog of the Scorpions of the World (1758–1998) New York, N.Y.: New York Entomological Society; 2000. pp. 54–286.
-
- Couraud F, Jover E. Mechanism of Action of Scorpion Toxins. In: Tu AT, editor. Handbook of Natural Toxins. New York, N.Y.: Marcel Dekker, Inc; 1984. pp. 659–678.
-
- Simard JM, Meves H, Watt DD. Neurotoxins in venom from the North American scorpion, Centruroides sculpturatus Ewing. In: Keeler RF, Mandava NB, Tu AT, editors. Natural Toxins: Toxicology, Chemistry and Safety. Alaken, Inc., Fort Collins, CO; 1992. pp. 236–263.
-
- Possani LD, Becerril B, Delepierre M, Tytgat J. Scorpion toxins specific for Na+-channels. European Journal of Biochemistry. 1999;264:287–300. - PubMed
-
- Possani LD, Merino E, Corona M, Bolivar F, Becerril B. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie. 2000;82:861–868. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

