Isolation and characterization of CvIV4: a pain inducing α-scorpion toxin
- PMID: 21887265
- PMCID: PMC3160894
- DOI: 10.1371/journal.pone.0023520
Isolation and characterization of CvIV4: a pain inducing α-scorpion toxin
Abstract
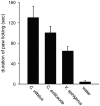
Background: Among scorpion species, the Buthidae produce the most deadly and painful venoms. However, little is known regarding the venom components that cause pain and their mechanism of action. Using a paw-licking assay (Mus musculus), this study compared the pain-inducing capabilities of venoms from two species of New World scorpion (Centruroides vittatus, C. exilicauda) belonging to the neurotoxin-producing family Buthidae with one species of non-neurotoxin producing scorpion (Vaejovis spinigerus) in the family Vaejovidae. A pain-inducing α-toxin (CvIV4) was isolated from the venom of C. vittatus and tested on five Na(+) channel isoforms.
Principal findings: C. vittatus and C. exilicauda venoms produced significantly more paw licking in Mus than V. spinigerus venom. CvIV4 produced paw licking in Mus equivalent to the effects of whole venom. CvIV4 slowed the fast inactivation of Na(v)1.7, a Na(+) channel expressed in peripheral pain-pathway neurons (nociceptors), but did not affect the Na(v)1.8-based sodium currents of these neurons. CvIV4 also slowed the fast inactivation of Na(v)1.2, Na(v)1.3 and Na(v)1.4. The effects of CvIV4 are similar to Old World α-toxins that target Na(v)1.7 (AahII, BmK MI, LqhIII, OD1), however the primary structure of CvIV4 is not similar to these toxins. Mutant Na(v)1.7 channels (D1586A and E1589Q, DIV S3-S4 linker) reduced but did not abolish the effects of CvIV4.
Conclusions: This study: 1) agrees with anecdotal evidence suggesting that buthid venom is significantly more painful than non-neurotoxic venom; 2) demonstrates that New World buthids inflict painful stings via toxins that modulate Na(+) channels expressed in nociceptors; 3) reveals that Old and New World buthids employ similar mechanisms to produce pain. Old and New World α-toxins that target Na(v)1.7 have diverged in sequence, but the activity of these toxins is similar. Pain-inducing toxins may have evolved in a common ancestor. Alternatively, these toxins may be the product of convergent evolution.
Conflict of interest statement
Figures




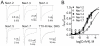
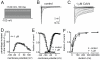



References
-
- Fet V, Lowe G. Family Buthidae. In: Fet V, Sissom WD, Lowe G, Braunwalder ME, editors. Catalog of the Scorpions of the World (1758–1998) New York, N.Y.: New York Entomological Society; 2000. pp. 54–286.
-
- Couraud F, Jover E. Mechanism of Action of Scorpion Toxins. In: Tu AT, editor. Handbook of Natural Toxins. New York, N.Y.: Marcel Dekker, Inc; 1984. pp. 659–678.
-
- Simard JM, Meves H, Watt DD. Neurotoxins in venom from the North American scorpion, Centruroides sculpturatus Ewing. In: Keeler RF, Mandava NB, Tu AT, editors. Natural Toxins: Toxicology, Chemistry and Safety. Alaken, Inc., Fort Collins, CO; 1992. pp. 236–263.
-
- Possani LD, Becerril B, Delepierre M, Tytgat J. Scorpion toxins specific for Na+-channels. European Journal of Biochemistry. 1999;264:287–300. - PubMed
-
- Possani LD, Merino E, Corona M, Bolivar F, Becerril B. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie. 2000;82:861–868. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

