In-cell NMR in E. coli to monitor maturation steps of hSOD1
- PMID: 21887272
- PMCID: PMC3160886
- DOI: 10.1371/journal.pone.0023561
In-cell NMR in E. coli to monitor maturation steps of hSOD1
Abstract
In-cell NMR allows characterizing the folding state of a protein as well as posttranslational events at molecular level, in the cellular context. Here, the initial maturation steps of human copper, zinc superoxide dismutase 1 are characterized in the E. coli cytoplasm by in-cell NMR: from the apo protein, which is partially unfolded, to the zinc binding which causes its final quaternary structure. The protein selectively binds only one zinc ion, whereas in vitro also the copper site binds a non-physiological zinc ion. However, no intramolecular disulfide bridge formation occurs, nor copper uptake, suggesting the need of a specific chaperone for those purposes.
Conflict of interest statement
Figures
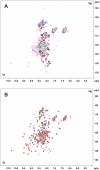

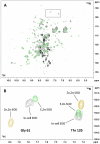
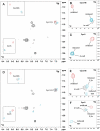
References
-
- Reckel S, Hänsel R, Löhr F, Dötsch V. In-cell NMR spectroscopy. Prog NMR Spectrosc. 2007;51:91–101.
-
- Selenko P, Wagner G. Looking into live cells with in-cell NMR spectroscopy. J Struct Biol. 2007;158:244–253. - PubMed
-
- Ritz D, Beckwith J. Roles of thiol-redox pathways in bacteria. Annu Rev Microbiol. 2001;55:21–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

