CK2 phosphorylation of Schistosoma mansoni HMGB1 protein regulates its cellular traffic and secretion but not its DNA transactions
- PMID: 21887276
- PMCID: PMC3160966
- DOI: 10.1371/journal.pone.0023572
CK2 phosphorylation of Schistosoma mansoni HMGB1 protein regulates its cellular traffic and secretion but not its DNA transactions
Abstract
Background: The helminth Schistosoma mansoni parasite resides in mesenteric veins where fecundated female worms lay hundred of eggs daily. Some of the egg antigens are trapped in the liver and induce a vigorous granulomatous response. High Mobility Group Box 1 (HMGB1), a nuclear factor, can also be secreted and act as a cytokine. Schistosome HMGB1 (SmHMGB1) is secreted by the eggs and stimulate the production of key cytokines involved in the pathology of schistosomiasis. Thus, understanding the mechanism of SmHMGB1 release becomes mandatory. Here, we addressed the question of how the nuclear SmHMGB1 can reach the extracellular space.
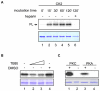
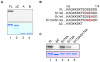
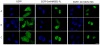
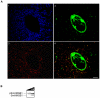
Principal findings: We showed in vitro and in vivo that CK2 phosphorylation was involved in the nucleocytoplasmic shuttling of SmHMGB1. By site-directed mutagenesis we mapped the two serine residues of SmHMGB1 that were phosphorylated by CK2. By DNA bending and supercoiling assays we showed that CK2 phosphorylation of SmHMGB1 had no effect in the DNA binding activities of the protein. We showed by electron microscopy, as well as by cell transfection and fluorescence microscopy that SmHMGB1 was present in the nucleus and cytoplasm of adult schistosomes and mammalian cells. In addition, we showed that treatments of the cells with either a phosphatase or a CK2 inhibitor were able to enhance or block, respectively, the cellular traffic of SmHMGB1. Importantly, we showed by confocal microscopy and biochemically that SmHMGB1 is significantly secreted by S. mansoni eggs of infected animals and that SmHMGB1 that were localized in the periovular schistosomotic granuloma were phosphorylated.
Conclusions: We showed that secretion of SmHMGB1 is regulated by phosphorylation. Moreover, our results suggest that egg-secreted SmHMGB1 may represent a new egg antigen. Therefore, the identification of drugs that specifically target phosphorylation of SmHMGB1 might block its secretion and interfere with the pathogenesis of schistosomiasis.
Conflict of interest statement
Figures




Similar articles
-
Cloning the genes and DNA binding properties of High Mobility Group B1 (HMGB1) proteins from the human blood flukes Schistosoma mansoni and Schistosoma japonicum.Gene. 2006 Aug 1;377:33-45. doi: 10.1016/j.gene.2006.03.001. Epub 2006 Apr 27. Gene. 2006. PMID: 16644144
-
The extracellular release of Schistosoma mansoni HMGB1 nuclear protein is mediated by acetylation.Biochem Biophys Res Commun. 2009 Dec 25;390(4):1245-9. doi: 10.1016/j.bbrc.2009.10.129. Epub 2009 Oct 29. Biochem Biophys Res Commun. 2009. PMID: 19879244
-
Cloning and characterization of a high mobility group box 1 (HMGB1) homologue protein from Schistosoma mansoni.Mol Biochem Parasitol. 2006 Feb;145(2):137-46. doi: 10.1016/j.molbiopara.2005.09.013. Epub 2005 Oct 7. Mol Biochem Parasitol. 2006. PMID: 16246438
-
Interaction and involvement of cellular adhesion molecules in the pathogenesis of Schistosomiasis mansoni.Immunol Lett. 2019 Feb;206:11-18. doi: 10.1016/j.imlet.2018.11.011. Epub 2018 Nov 29. Immunol Lett. 2019. PMID: 30503821 Review.
-
T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis.Front Immunol. 2020 Feb 18;11:61. doi: 10.3389/fimmu.2020.00061. eCollection 2020. Front Immunol. 2020. PMID: 32132991 Free PMC article. Review.
Cited by
-
HMGA1, Moonlighting Protein Function, and Cellular Real Estate: Location, Location, Location!Biomolecules. 2021 Sep 9;11(9):1334. doi: 10.3390/biom11091334. Biomolecules. 2021. PMID: 34572547 Free PMC article. Review.
-
Protein kinase CK2: a potential therapeutic target for diverse human diseases.Signal Transduct Target Ther. 2021 May 17;6(1):183. doi: 10.1038/s41392-021-00567-7. Signal Transduct Target Ther. 2021. PMID: 33994545 Free PMC article. Review.
-
HMGB1 in health and disease.Mol Aspects Med. 2014 Dec;40:1-116. doi: 10.1016/j.mam.2014.05.001. Epub 2014 Jul 8. Mol Aspects Med. 2014. PMID: 25010388 Free PMC article. Review.
-
The dengue vector Aedes aegypti contains a functional high mobility group box 1 (HMGB1) protein with a unique regulatory C-terminus.PLoS One. 2012;7(7):e40192. doi: 10.1371/journal.pone.0040192. Epub 2012 Jul 3. PLoS One. 2012. PMID: 22802955 Free PMC article.
-
Emerging Role of HMGB1 in the Pathogenesis of Schistosomiasis Liver Fibrosis.Front Immunol. 2018 Sep 12;9:1979. doi: 10.3389/fimmu.2018.01979. eCollection 2018. Front Immunol. 2018. PMID: 30258438 Free PMC article.
References
-
- WHO. TDR Strategic Direction for Research. Schistosomiasis World Health Organization. Geneve; 2002.
-
- Oliveira VR, El-Cheikh MC, Aguiar AM, Balduino A, de Fatima BPM, et al. Schistosoma mansoni egg-induced hepatic granulomas in mice deficient for the interferon-gamma receptor have altered populations of macrophages, lymphocytes and connective tissue cells. Microbes Infect. 2000;2:1817–1826. - PubMed
-
- Boros DL. Immunoregulation of granuloma formation in murine schistosomiasis mansoni. Ann N Y Acad Sci. 1986;465:313–323. - PubMed
-
- Caldas IR, Campi-Azevedo AC, Oliveira LF, Silveira AM, Oliveira RC, et al. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Trop. 2008;108:109–117. - PubMed
-
- Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010;1799:101–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

