Serum-nutrient starvation induces cell death mediated by Bax and Puma that is counteracted by p21 and unmasked by Bcl-x(L) inhibition
- PMID: 21887277
- PMCID: PMC3160893
- DOI: 10.1371/journal.pone.0023577
Serum-nutrient starvation induces cell death mediated by Bax and Puma that is counteracted by p21 and unmasked by Bcl-x(L) inhibition
Abstract
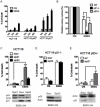
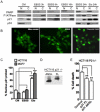
The cyclin-dependent kinase inhibitor p21 (p21WAF1/Cip1) is a multifunctional protein known to promote cell cycle arrest and survival in response to p53-dependent and p53 independent stimuli. We herein investigated whether and how it might contribute to the survival of cancer cells that are in low-nutrient conditions during tumour growth, by culturing isogenic human colorectal cancer cell lines (HCT116) and breast cancer cell lines in a medium deprived in amino acids and serum. We show that such starvation enhances, independently from p53, the expression of p21 and that of the pro-apoptotic BH3-only protein Puma. Under these conditions, p21 prevents Puma and its downstream effector Bax from triggering the mitochondrial apoptotic pathway. This anti-apoptotic effect is exerted from the cytosol but it is unrelated to the ability of p21 to interfere with the effector caspase 3. The survival function of p21 is, however, overcome by RNA interference mediated Bcl-x(L) depletion, or by the pharmacological inhibitor ABT-737. Thus, an insufficient supply in nutrients may not have an overt effect on cancer cell viability due to p21 induction, but it primes these cells to die, and sensitizes them to the deleterious effects of Bcl-x(L) inhibitors regardless of their p53 status.
Conflict of interest statement
Figures




References
-
- Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–49. - PubMed
-
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. - PubMed
-
- Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13:65–70. - PubMed
-
- Janicke RU, Sohn D, Essmann F, Schulze-Osthoff K. The multiple battles fought by anti-apoptotic p21. Cell Cycle. 2007;6:407–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

