Rectification of the water permeability in COS-7 cells at 22, 10 and 0°C
- PMID: 21887290
- PMCID: PMC3161049
- DOI: 10.1371/journal.pone.0023643
Rectification of the water permeability in COS-7 cells at 22, 10 and 0°C
Abstract
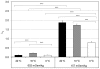
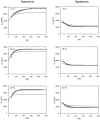
The osmotic and permeability parameters of a cell membrane are essential physico-chemical properties of a cell and particularly important with respect to cell volume changes and the regulation thereof. Here, we report the hydraulic conductivity, L(p), the non-osmotic volume, V(b), and the Arrhenius activation energy, E(a), of mammalian COS-7 cells. The ratio of V(b) to the isotonic cell volume, V(c iso), was 0.29. E(a), the activation energy required for the permeation of water through the cell membrane, was 10,700, and 12,000 cal/mol under hyper- and hypotonic conditions, respectively. Average values for L(p) were calculated from swell/shrink curves by using an integrated equation for L(p). The curves represented the volume changes of 358 individually measured cells, placed into solutions of nonpermeating solutes of 157 or 602 mOsm/kg (at 0, 10 or 22°C) and imaged over time. L(p) estimates for all six combinations of osmolality and temperature were calculated, resulting in values of 0.11, 0.21, and 0.10 µm/min/atm for exosmotic flow and 0.79, 1.73 and 1.87 µm/min/atm for endosmotic flow (at 0, 10 and 22°C, respectively). The unexpected finding of several fold higher L(p) values for endosmotic flow indicates highly asymmetric membrane permeability for water in COS-7. This phenomenon is known as rectification and has mainly been reported for plant cell, but only rarely for animal cells. Although the mechanism underlying the strong rectification found in COS-7 cells is yet unknown, it is a phenomenon of biological interest and has important practical consequences, for instance, in the development of optimal cryopreservation.
Conflict of interest statement
Figures





References
-
- Mazur P. Principles of Cryobiology. In: Fuller NLBJ, Benson EE, editors. Life in the Frozen State. Boca Raton: CRC Press; 2004. pp. 3–65.
-
- Mazur P, Seki S, Pinn IL, Kleinhans FW, Edashige K. Extra- and intracellular ice formation in mouse oocytes. Cryobiology. 2005;51:29–53. - PubMed
-
- Kleinhans FW, Guenther JF, Roberts DM, Mazur P. Analysis of intracellular ice nucleation in Xenopus oocytes by differential scanning calorimetry. Cryobiology. 2006;52:128–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

