Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans
- PMID: 21887302
- PMCID: PMC3161750
- DOI: 10.1371/journal.pone.0023710
Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans
Abstract
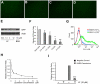
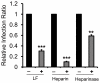
It has been reported that lactoferrin (LF) participates in the host immune response against Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) invasion by enhancing NK cell activity and stimulating neutrophil aggregation and adhesion. We further investigated the role of LF in the entry of SARS pseudovirus into HEK293E/ACE2-Myc cells. Our results reveal that LF inhibits SARS pseudovirus infection in a dose-dependent manner. Further analysis suggested that LF was able to block the binding of spike protein to host cells at 4°C, indicating that LF exerted its inhibitory function at the viral attachment stage. However, LF did not disrupt the interaction of spike protein with angiotensin-converting enzyme 2 (ACE2), the functional receptor of SARS-CoV. Previous studies have shown that LF colocalizes with the widely distributed cell-surface heparan sulfate proteoglycans (HSPGs). Our experiments have also confirmed this conclusion. Treatment of the cells with heparinase or exogenous heparin prevented binding of spike protein to host cells and inhibited SARS pseudovirus infection, demonstrating that HSPGs provide the binding sites for SARS-CoV invasion at the early attachment phase. Taken together, our results suggest that, in addition to ACE2, HSPGs are essential cell-surface molecules involved in SARS-CoV cell entry. LF may play a protective role in host defense against SARS-CoV infection through binding to HSPGs and blocking the preliminary interaction between SARS-CoV and host cells. Our findings may provide further understanding of SARS-CoV pathogenesis and aid in treatment of this deadly disease.
Conflict of interest statement
Figures
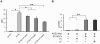

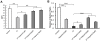


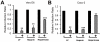

References
-
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. - PubMed
-
- Li F, Li W, Farzan M, Harrison SC. Structure of SARS Coronavirus Spike Receptor-Binding Domain Complexed with Receptor. Science. 2005;309:1864–1868. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

