Efficient detection of proteins retro-translocated from the ER to the cytosol by in vivo biotinylation
- PMID: 21887304
- PMCID: PMC3161056
- DOI: 10.1371/journal.pone.0023712
Efficient detection of proteins retro-translocated from the ER to the cytosol by in vivo biotinylation
Abstract
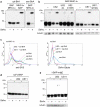
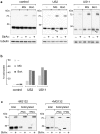
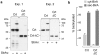
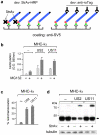
Retro-translocation from the ER to the cytosol of proteins within the secretory pathway takes place on misfolded molecules that are targeted for degradation by the cytosolically located 26S proteasome complex. Retro-translocation occurs also for other proteins (such as calreticulin) that, despite being synthesized and transported to the ER, are in part dislocated to the cytosol. We have taken advantage of the E. coli derived biotin-ligase (BirA) expressed in the cytosol of mammalian cells to specifically biotin-label in vivo proteins within the secretory pathway that undergo retro-translocation. We validated the method using four different proteins that are known to undergo retro-translocation upon different conditions: the human trans-membrane protein MHC class-I α chain (MHC-Iα), the Null Hong Kong mutant of the secretory α1 anti-trypsin (NHK-α1AT), the immunoglobulin heavy chain (HC) and the ER chaperone calreticulin (Crt). We observed specific mono-biotinylation of cytosolically dislocated molecules, resulting in a novel, reliable way of determining the extent of retro-translocation.
Conflict of interest statement
Figures



Similar articles
-
Application of the biotin-labeled toxin mutant for affinity isolation of associated proteins in the mammalian cells.J Biosci Bioeng. 2018 May;125(5):497-504. doi: 10.1016/j.jbiosc.2017.12.002. Epub 2017 Dec 29. J Biosci Bioeng. 2018. PMID: 29291913
-
Metabolic biotinylation of recombinant antibody by biotin ligase retained in the endoplasmic reticulum.Biomol Eng. 2007 Sep;24(3):283-91. doi: 10.1016/j.bioeng.2007.02.003. Epub 2007 Feb 15. Biomol Eng. 2007. PMID: 17379573 Free PMC article.
-
Ubiquitination is required for the retro-translocation of a short-lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome.J Biol Chem. 1998 Apr 17;273(16):9734-43. doi: 10.1074/jbc.273.16.9734. J Biol Chem. 1998. PMID: 9545309
-
Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum.Toxins (Basel). 2010 Mar;2(3):310-25. doi: 10.3390/toxins2030310. Epub 2010 Mar 5. Toxins (Basel). 2010. PMID: 22069586 Free PMC article. Review.
-
Retro-translocation of proteins from the endoplasmic reticulum into the cytosol.Nat Rev Mol Cell Biol. 2002 Apr;3(4):246-55. doi: 10.1038/nrm780. Nat Rev Mol Cell Biol. 2002. PMID: 11994744 Review.
Cited by
-
Intracellular complement: Evidence, definitions, controversies, and solutions.Immunol Rev. 2023 Jan;313(1):104-119. doi: 10.1111/imr.13135. Epub 2022 Sep 13. Immunol Rev. 2023. PMID: 36100972 Free PMC article. Review.
-
Selective targeting of proteins within secretory pathway for endoplasmic reticulum-associated degradation.J Biol Chem. 2012 Jun 8;287(24):20007-15. doi: 10.1074/jbc.M112.355107. Epub 2012 Apr 20. J Biol Chem. 2012. PMID: 22523070 Free PMC article.
-
Cytosolic entry of Shiga-like toxin a chain from the yeast endoplasmic reticulum requires catalytically active Hrd1p.PLoS One. 2012;7(7):e41119. doi: 10.1371/journal.pone.0041119. Epub 2012 Jul 19. PLoS One. 2012. PMID: 22829918 Free PMC article.
-
Familial prion protein mutants inhibit Hrd1-mediated retrotranslocation of misfolded proteins by depleting misfolded protein sensor BiP.Hum Mol Genet. 2016 Mar 1;25(5):976-88. doi: 10.1093/hmg/ddv630. Epub 2016 Jan 5. Hum Mol Genet. 2016. PMID: 26740554 Free PMC article.
-
The VCP/p97 and YOD1 Proteins Have Different Substrate-dependent Activities in Endoplasmic Reticulum-associated Degradation (ERAD).J Biol Chem. 2015 Nov 20;290(47):28175-28188. doi: 10.1074/jbc.M115.656660. Epub 2015 Oct 13. J Biol Chem. 2015. PMID: 26463207 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

