Ischemic tolerance protects the rat retina from glaucomatous damage
- PMID: 21887313
- PMCID: PMC3161053
- DOI: 10.1371/journal.pone.0023763
Ischemic tolerance protects the rat retina from glaucomatous damage
Abstract
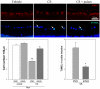
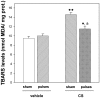
Glaucoma is a leading cause of acquired blindness which may involve an ischemic-like insult to retinal ganglion cells and optic nerve head. We investigated the effect of a weekly application of brief ischemia pulses (ischemic conditioning) on the rat retinal damage induced by experimental glaucoma. Glaucoma was induced by weekly injections of chondroitin sulfate (CS) in the rat eye anterior chamber. Retinal ischemia was induced by increasing intraocular pressure to 120 mmHg for 5 min; this maneuver started after 6 weekly injections of vehicle or CS and was weekly repeated in one eye, while the contralateral eye was submitted to a sham procedure. Glaucoma was evaluated in terms of: i) intraocular pressure (IOP), ii) retinal function (electroretinogram (ERG)), iii) visual pathway function (visual evoked potentials, (VEPs)) iv) histology of the retina and optic nerve head. Retinal thiobarbituric acid substances levels were assessed as an index of lipid peroxidation. Ischemic conditioning significantly preserved ERG, VEPs, as well as retinal and optic nerve head structure from glaucomatous damage, without changes in IOP. Moreover, ischemia pulses abrogated the increase in lipid peroxidation induced by experimental glaucoma. These results indicate that induction of ischemic tolerance could constitute a fertile avenue for the development of new therapeutic strategies in glaucoma treatment.
Conflict of interest statement
Figures





References
-
- Belforte N, Sande P, de Zavalía N, Knepper PA, Rosenstein RE. Effect of chondroitin sulfate on intraocular pressure in rats. Invest Ophthalmol Vis Sci. 2010;51:5768–5775. - PubMed
-
- Chung HS, Harris A, Evans DW, Kagemann L, Garzozi HJ, et al. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43:S43–50. - PubMed
-
- Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38:S3–6. - PubMed
-
- Delaney Y, Walshe TE, O'Brien C. Vasospasm in glaucoma: clinical and laboratory aspects. Optom Vis Sci. 2006;83:406–414. - PubMed
-
- Gugleta K. Vascular risk factors in glaucoma – diagnostics. Praxis (Bern 1994) 2009;98:201–207. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

