Laforin, a dual specificity phosphatase involved in Lafora disease, is present mainly as monomeric form with full phosphatase activity
- PMID: 21887368
- PMCID: PMC3162602
- DOI: 10.1371/journal.pone.0024040
Laforin, a dual specificity phosphatase involved in Lafora disease, is present mainly as monomeric form with full phosphatase activity
Abstract
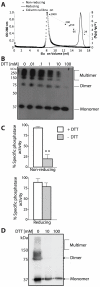
Lafora Disease (LD) is a fatal neurodegenerative epileptic disorder that presents as a neurological deterioration with the accumulation of insoluble, intracellular, hyperphosphorylated carbohydrates called Lafora bodies (LBs). LD is caused by mutations in either the gene encoding laforin or malin. Laforin contains a dual specificity phosphatase domain and a carbohydrate-binding module, and is a member of the recently described family of glucan phosphatases. In the current study, we investigated the functional and physiological relevance of laforin dimerization. We purified recombinant human laforin and subjected the monomer and dimer fractions to denaturing gel electrophoresis, mass spectrometry, phosphatase assays, protein-protein interaction assays, and glucan binding assays. Our results demonstrate that laforin prevalently exists as a monomer with a small dimer fraction both in vitro and in vivo. Of mechanistic importance, laforin monomer and dimer possess equal phosphatase activity, and they both associate with malin and bind glucans to a similar extent. However, we found differences between the two states' ability to interact simultaneously with malin and carbohydrates. Furthermore, we tested other members of the glucan phosphatase family. Cumulatively, our data suggest that laforin monomer is the dominant form of the protein and that it contains phosphatase activity.
Conflict of interest statement
Figures





Similar articles
-
Dimerization of the glucan phosphatase laforin requires the participation of cysteine 329.PLoS One. 2013 Jul 26;8(7):e69523. doi: 10.1371/journal.pone.0069523. Print 2013. PLoS One. 2013. PMID: 23922729 Free PMC article.
-
A bioassay for Lafora disease and laforin glucan phosphatase activity.Clin Biochem. 2013 Dec;46(18):1869-76. doi: 10.1016/j.clinbiochem.2013.08.016. Epub 2013 Sep 6. Clin Biochem. 2013. PMID: 24012855 Free PMC article.
-
Impaired malin expression and interaction with partner proteins in Lafora disease.J Biol Chem. 2024 May;300(5):107271. doi: 10.1016/j.jbc.2024.107271. Epub 2024 Apr 7. J Biol Chem. 2024. PMID: 38588813 Free PMC article.
-
Laforin, a protein with many faces: glucan phosphatase, adapter protein, et alii.FEBS J. 2013 Jan;280(2):525-37. doi: 10.1111/j.1742-4658.2012.08549.x. Epub 2012 Mar 16. FEBS J. 2013. PMID: 22364389 Free PMC article. Review.
-
Deciphering the role of malin in the lafora progressive myoclonus epilepsy.IUBMB Life. 2012 Oct;64(10):801-8. doi: 10.1002/iub.1072. Epub 2012 Jul 20. IUBMB Life. 2012. PMID: 22815132 Free PMC article. Review.
Cited by
-
Structural mechanism of laforin function in glycogen dephosphorylation and lafora disease.Mol Cell. 2015 Jan 22;57(2):261-72. doi: 10.1016/j.molcel.2014.11.020. Epub 2014 Dec 24. Mol Cell. 2015. PMID: 25544560 Free PMC article.
-
Assessing the Biological Activity of the Glucan Phosphatase Laforin.Methods Mol Biol. 2016;1447:107-19. doi: 10.1007/978-1-4939-3746-2_7. Methods Mol Biol. 2016. PMID: 27514803 Free PMC article.
-
Dimeric quaternary structure of human laforin.J Biol Chem. 2015 Feb 20;290(8):4552-4559. doi: 10.1074/jbc.M114.627406. Epub 2014 Dec 23. J Biol Chem. 2015. PMID: 25538239 Free PMC article.
-
Dimerization of the glucan phosphatase laforin requires the participation of cysteine 329.PLoS One. 2013 Jul 26;8(7):e69523. doi: 10.1371/journal.pone.0069523. Print 2013. PLoS One. 2013. PMID: 23922729 Free PMC article.
-
Identification and analysis of OsttaDSP, a phosphoglucan phosphatase from Ostreococcus tauri.PLoS One. 2018 Jan 23;13(1):e0191621. doi: 10.1371/journal.pone.0191621. eCollection 2018. PLoS One. 2018. PMID: 29360855 Free PMC article.
References
-
- Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet. 1998;20:171–174. - PubMed
-
- Serratosa JM, Gomez-Garre P, Gallardo ME, Anta B, de Bernabe DB, et al. A novel protein tyrosine phosphatase gene is mutated in progressive myoclonus epilepsy of the Lafora type (EPM2). Hum Mol Genet. 1999;8:345–352. - PubMed
-
- Berkovic SF, So NK, Andermann F. Progressive myoclonus epilepsies: clinical and neurophysiological diagnosis. J Clin Neurophysiol. 1991;8:261–274. - PubMed
-
- Janeway R, Ravens JR, Pearce LA, Odor DL, Suzuki K. Progressive myoclonus epilepsy with Lafora inclusion bodies. I. Clinical, genetic, histopathologic, and biochemical aspects. Arch Neurol. 1967;16:565–582. - PubMed
-
- Van Heycop Ten Ham MW. Lafora disease, a form of progressive myoclonus epilepsy. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical neurology. Amsterdam, Holland: North Holland Publishing Company; 1975. pp. 382–422.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

