Nuclear sphingolipid metabolism
- PMID: 21888508
- PMCID: PMC3981831
- DOI: 10.1146/annurev-physiol-020911-153321
Nuclear sphingolipid metabolism
Abstract
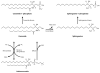
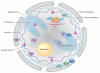
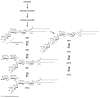
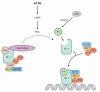
Nuclear lipid metabolism is implicated in various processes, including transcription, splicing, and DNA repair. Sphingolipids play roles in numerous cellular functions, and an emerging body of literature has identified roles for these lipid mediators in distinct nuclear processes. Different sphingolipid species are localized in various subnuclear domains, including chromatin, the nuclear matrix, and the nuclear envelope, where sphingolipids exert specific regulatory and structural functions. Sphingomyelin, the most abundant nuclear sphingolipid, plays both structural and regulatory roles in chromatin assembly and dynamics in addition to being an integral component of the nuclear matrix. Sphingosine-1-phosphate modulates histone acetylation, sphingosine is a ligand for steroidogenic factor 1, and nuclear accumulation of ceramide has been implicated in apoptosis. Finally, nuclear membrane-associated ganglioside GM1 plays a pivotal role in Ca(2+) homeostasis. This review highlights research on the factors that control nuclear sphingolipid metabolism and summarizes the roles of these lipids in various nuclear processes.
Figures

Similar articles
-
Gene expression of sphingolipid metabolism pathways is altered in hidradenitis suppurativa.J Am Acad Dermatol. 2017 Aug;77(2):268-273.e6. doi: 10.1016/j.jaad.2017.03.016. Epub 2017 May 24. J Am Acad Dermatol. 2017. PMID: 28551069
-
Involvement of sphingolipids metabolites in cellular proliferation modulated by ganglioside GM1.Glycoconj J. 1996 Dec;13(6):937-45. doi: 10.1007/BF01053189. Glycoconj J. 1996. PMID: 8981085
-
Impact of Sphingolipid Mediators on the Determination of Cochlear Survival in Ototoxicity.Curr Mol Pharmacol. 2018;11(4):279-284. doi: 10.2174/1874467211666180516101111. Curr Mol Pharmacol. 2018. PMID: 29766830 Review.
-
An overview of sphingolipid metabolism: from synthesis to breakdown.Adv Exp Med Biol. 2010;688:1-23. doi: 10.1007/978-1-4419-6741-1_1. Adv Exp Med Biol. 2010. PMID: 20919643 Free PMC article. Review.
-
Nuclear lipid mediators: Role of nuclear sphingolipids and sphingosine-1-phosphate signaling in epigenetic regulation of inflammation and gene expression.J Cell Biochem. 2018 Aug;119(8):6337-6353. doi: 10.1002/jcb.26707. Epub 2018 May 8. J Cell Biochem. 2018. PMID: 29377310 Free PMC article. Review.
Cited by
-
Lipidomics of Alzheimer's disease: current status.Alzheimers Res Ther. 2012 Feb 1;4(1):5. doi: 10.1186/alzrt103. Alzheimers Res Ther. 2012. PMID: 22293144 Free PMC article.
-
ATP-Binding Cassette Transporter Family C Protein 10 Participates in the Synthesis and Efflux of Hexosylceramides in Liver Cells.Nutrients. 2022 Oct 20;14(20):4401. doi: 10.3390/nu14204401. Nutrients. 2022. PMID: 36297086 Free PMC article.
-
Molecular Mechanisms of Sphingolipid Transport on Plasma Lipoproteins.Adv Exp Med Biol. 2022;1372:57-65. doi: 10.1007/978-981-19-0394-6_5. Adv Exp Med Biol. 2022. PMID: 35503174
-
Adjuvant treatment with monosialoganglioside may improve neurological outcomes in neonatal hypoxic-ischemic encephalopathy: A meta-analysis of randomized controlled trials.PLoS One. 2017 Aug 23;12(8):e0183490. doi: 10.1371/journal.pone.0183490. eCollection 2017. PLoS One. 2017. PMID: 28832625 Free PMC article.
-
GM1 Ganglioside: Past Studies and Future Potential.Mol Neurobiol. 2016 Apr;53(3):1824-1842. doi: 10.1007/s12035-015-9136-z. Epub 2015 Mar 12. Mol Neurobiol. 2016. PMID: 25762012 Review.
References
-
- Ledeen RW, Wu G. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim. Biophys. Acta. 2006;1761:588–98. - PubMed
-
- Tamiya-Koizumi K. Nuclear lipid metabolism and signaling. J. Biochem. 2002;132:13–22. - PubMed
-
- Albi E, Viola Magni M. The role of intranuclear lipids. Biol. Cell. 2004;96:657–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

