Esmolol modulates inhibitory neurotransmission in the substantia gelatinosa of the spinal trigeminal nucleus of the rat
- PMID: 21888677
- PMCID: PMC3175182
- DOI: 10.1186/1471-2253-11-15
Esmolol modulates inhibitory neurotransmission in the substantia gelatinosa of the spinal trigeminal nucleus of the rat
Abstract
Background: β1-adrenaline receptor antagonists are often used to avoid circulatory complications during anesthesia in patients with cardiovascular diseases. Of these drugs, esmolol, a short-acting β antagonist, is also reported to exert antinociceptive and anesthetic sparing effects. This study was designed to identify the central mechanism underlying the antinociceptive effect of esmolol.
Methods: Wistar rats (7-21 d, 17-50 g) were anesthetized with ketamine (100-150 mg/kg) or isoflurane (5%) and decapitated. Horizontal slices (400-μm thick) of the lower brainstem containing the substantia gelatinosa (SG) of the caudal part of the spinal trigeminal nucleus (Sp5c), in which the nociceptive primary afferents form the first intracranial synapses, were made with a vibrating slicer. The miniature inhibitory and excitatory postsynaptic currents (mIPSCs and mEPSCs, respectively) were simultaneously recorded from visually identified SG neurons of the Sp5c in the presence of tetrodotoxin (1 μM). Additionally, mIPSCs were recorded during pharmacological isolation of GABA- and glycine-mediated mIPSCs with kynurenic acid (1 mM).
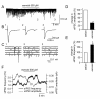
Results: Esmolol (500 μM) significantly and selectively increased the mIPSC frequency (to 214.2% ± 34.2% of the control, mean ± SEM, n = 35; P < 0.001), but not that of mEPSCs, without changing their amplitude. The increase in mIPSC frequency with esmolol was not affected by prior activation of β receptors with isoproterenol (100 μM) but it was significantly attenuated by removal of extracellular Ca2+.
Conclusions: These data suggest that esmolol modulates inhibitory transmitter release in the Sp5c through a mechanism involving Ca2+-entry but in a β1-adrenoceptor-independent manner. The present results suggest that the facilitation of inhibitory transmitter release in the central nociceptive network underlies, at least in part, the antinociceptive effect of esmolol.
Figures



References
-
- Johansen JW, Schneider G, Windsor AM, Sebel PS. Esmolol potentiates reduction of minimum alveolar isoflurane concentration by alfentanil. Anesth Analg. 1998;87:671–6. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

