T-helper cells as new players in ANCA-associated vasculitides
- PMID: 21888687
- PMCID: PMC3239339
- DOI: 10.1186/ar3362
T-helper cells as new players in ANCA-associated vasculitides
Abstract
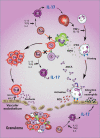
In anti-neutrophil cytoplasmic autoantibody-associated vasculitides (AAV), several observations support a key role of T-helper cells (CD4(+) T cells) in disease pathophysiology. An expanded population of effector memory CD4(+) T cells in AAV patients may contribute to tissue injury and disease progression. In addition, functional impairment of regulatory T cells (T(Regs)) is reported in AAV patients. A fraction of T(Regs) have the capacity to differentiate into Th17 cells in the context of a proinflammatory environment. Therefore, nonfunctionality of T(Regs) described in AAV patients may be caused by their conversion into IL-17-producing cells that may contribute to granulomatous vasculitis. Further investigations directed at the plasticity of T(Regs) in AAV patients are warranted.
Figures
References
-
- Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol. 2004;93:398–401. doi: 10.1016/S1081-1206(10)61400-7. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

