N-acetylcysteine protects pancreatic islet against glucocorticoid toxicity
- PMID: 21888768
- PMCID: PMC6837713
- DOI: 10.1179/1351000211Y.0000000006
N-acetylcysteine protects pancreatic islet against glucocorticoid toxicity
Abstract
Objectives: Reactive oxygen species (ROS) are involved in many physiological and pathological processes. In the present study, we analysed whether the synthetic glucocorticoid dexamethasone induces oxidative stress in cultured pancreatic islets and whether the effects of dexamethasone on insulin secretion, gene expression, and viability can be counteracted by concomitant incubation with N-acetylcysteine (NAC).
Methods: ROS production was measured by dichlorofluorescein (DCFH-DA) assay, insulin secretion by radioimmunoassay, intracellular calcium dynamics by fura-2-based fluorescence, gene expression by real-time polymerase chain reaction analyses and cell viability by the MTS assay.
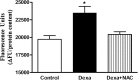
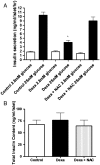
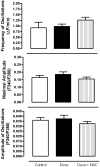
Results: Dexamethasone (Dexa) increased ROS production and decreased glucose-stimulated insulin secretion after 72 hours incubation. Intracellular ROS levels were decreased and the insulin secretion capacity was recovered by concomitant treatment with Dexa+NAC. The total insulin content and intracellular Ca2+ levels were not modulated in either Dexa or Dexa+NAC groups. There was a decrease in the NAD(P)H production, used as an indicator of viability, after dexamethasone treatment. Concomitant incubation with NAC returned viability to control levels. Dexa also decreased synaptotagmin VII (SYT VII) gene expression. In contrast, the Dexa+NAC group demonstrated an increased expression of SYT VII compared to controls. Surprisingly, treatment with NAC decreased the gene expression of the antioxidant enzyme copper zinc superoxide dismutase soluble.
Discussion: Our results indicate that dexamethasone increases ROS production, decreases viability, and impairs insulin secretion in pancreatic rat islets. These effects can be counteracted by NAC, which not only decreases ROS levels but also modulates the expression of genes involved in the secretory pathway and those coding for antioxidant enzymes.
Figures


Similar articles
-
Glucose-Dependent Insulin Secretion in Pancreatic β-Cell Islets from Male Rats Requires Ca2+ Release via ROS-Stimulated Ryanodine Receptors.PLoS One. 2015 Jun 5;10(6):e0129238. doi: 10.1371/journal.pone.0129238. eCollection 2015. PLoS One. 2015. PMID: 26046640 Free PMC article.
-
Src activation generates reactive oxygen species and impairs metabolism-secretion coupling in diabetic Goto-Kakizaki and ouabain-treated rat pancreatic islets.Diabetologia. 2008 Jul;51(7):1226-35. doi: 10.1007/s00125-008-1008-x. Epub 2008 May 1. Diabetologia. 2008. PMID: 18449527
-
Preconditioning with millimolar concentrations of vitamin C or N-acetylcysteine protects L6 muscle cells insulin-stimulated viability and DNA synthesis under oxidative stress.Life Sci. 2002 Aug 30;71(15):1793-808. doi: 10.1016/s0024-3205(02)01942-2. Life Sci. 2002. PMID: 12151057
-
[N-acetyl-l-cysteine improves function of islet beta cell in hyperlipidemic rats and its mechanism].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2007 Nov;36(6):575-80. doi: 10.3785/j.issn.1008-9292.2007.06.010. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2007. PMID: 18067231 Chinese.
-
N-acetylcysteine and neurodegenerative diseases: basic and clinical pharmacology.Cerebellum. 2007;6(4):308-14. doi: 10.1080/14734220601142878. Epub 2007 Jan 19. Cerebellum. 2007. PMID: 17853088 Free PMC article. Review.
Cited by
-
Complexity of NAC Action as an Antidiabetic Agent: Opposing Effects of Oxidative and Reductive Stress on Insulin Secretion and Insulin Signaling.Int J Mol Sci. 2022 Mar 9;23(6):2965. doi: 10.3390/ijms23062965. Int J Mol Sci. 2022. PMID: 35328386 Free PMC article.
-
Redox Balance in Type 2 Diabetes: Therapeutic Potential and the Challenge of Antioxidant-Based Therapy.Antioxidants (Basel). 2023 Apr 25;12(5):994. doi: 10.3390/antiox12050994. Antioxidants (Basel). 2023. PMID: 37237860 Free PMC article. Review.
-
In vitro comparison of various antioxidants and flavonoids from Rooibos as beta cell protectants against lipotoxicity and oxidative stress-induced cell death.PLoS One. 2022 May 17;17(5):e0268551. doi: 10.1371/journal.pone.0268551. eCollection 2022. PLoS One. 2022. PMID: 35580081 Free PMC article.
-
Underlying mechanisms of glucocorticoid-induced β-cell death and dysfunction: a new role for glycogen synthase kinase 3.Cell Death Dis. 2021 Dec 7;12(12):1136. doi: 10.1038/s41419-021-04419-8. Cell Death Dis. 2021. PMID: 34876563 Free PMC article.
-
Channel Expansion in the Ligand-Binding Domain of the Glucocorticoid Receptor Contributes to the Activity of Highly Potent Glucocorticoid Analogues.Molecules. 2024 Mar 29;29(7):1546. doi: 10.3390/molecules29071546. Molecules. 2024. PMID: 38611825 Free PMC article.
References
-
- Lenzen S. Oxidative stress: the vulnerable beta-cell. Biochem Soc Trans 2008;36:343–7. - PubMed
-
- Souza KL, Elsner M, Mathias PC, Lenzen S, Tiedge M. Cytokines activate genes of the endocytotic pathway in insulin-producing RINm5F cells. Diabetologia 2004;47:1292–302. - PubMed
-
- Souza KL, Gurgul-Convey E, Elsner M, Lenzen S. Interaction between pro-inflammatory and anti-inflammatory cytokines in insulin-producing cells. J Endocrinol 2008;197:139–50. - PubMed
-
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996;20:463–6. - PubMed
-
- Li N, Frigerio F, Maechler P. The sensitivity of pancreatic beta-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem Soc Trans 2008;36:930–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
