Estimating effect of antiviral drug use during pandemic (H1N1) 2009 outbreak, United States
- PMID: 21888783
- PMCID: PMC3358088
- DOI: 10.3201/eid1709.110295
Estimating effect of antiviral drug use during pandemic (H1N1) 2009 outbreak, United States
Abstract
From April 2009 through March 2010, during the pandemic (H1N1) 2009 outbreak, ≈8.2 million prescriptions for influenza neuraminidase-inhibiting antiviral drugs were filled in the United States. We estimated the number of hospitalizations likely averted due to use of these antiviral medications. After adjusting for prescriptions that were used for prophylaxis and personal stockpiles, as well as for patients who did not complete their drug regimen, we estimated the filled prescriptions prevented ≈8,400-12,600 hospitalizations (on the basis of median values). Approximately 60% of these prevented hospitalizations were among adults 18-64 years of age, with the remainder almost equally divided between children 0-17 years of age and adults >65 years of age. Public health officials should consider these estimates an indication of success of treating patients during the 2009 pandemic and a warning of the need for renewed planning to cope with the next pandemic.
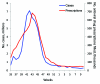
Figures
References
-
- Centers for Disease Control and Prevention. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season [cited 2010 Dec 18]. http://www.cdc.gov/H1N1flu/recommendations.htm#d
-
- Health IMS. Data Assets, IMS Prescription Data [cited 2010 Dec 18]. http://www.imshealth.com/portal/site/imshealth/menuitem.a46c6d4df3db4b3d...
-
- Strong M, Burrows J, Stedman E, Redgrave P. Adverse drug effects following oseltamivir mass treatment and prophylaxis in a school outbreak of 2009 pandemic influenza A(H1N1) in June 2009, Sheffield, United Kingdom. Euro Surveill 2010;15:pii:19565. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
