Calcium-calmodulin dependent protein kinase II (CaMKII): a main signal responsible for early reperfusion arrhythmias
- PMID: 21888910
- PMCID: PMC3208750
- DOI: 10.1016/j.yjmcc.2011.08.010
Calcium-calmodulin dependent protein kinase II (CaMKII): a main signal responsible for early reperfusion arrhythmias
Abstract
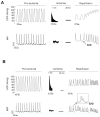
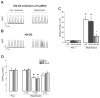
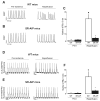
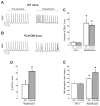
To explore whether CaMKII-dependent phosphorylation events mediate reperfusion arrhythmias, Langendorff perfused hearts were submitted to global ischemia/reperfusion. Epicardial monophasic or transmembrane action potentials and contractility were recorded. In rat hearts, reperfusion significantly increased the number of premature beats (PBs) relative to pre-ischemic values. This arrhythmic pattern was associated with a significant increase in CaMKII-dependent phosphorylation of Ser2814 on Ca(2+)-release channels (RyR2) and Thr17 on phospholamban (PLN) at the sarcoplasmic reticulum (SR). These phenomena could be prevented by the CaMKII-inhibitor KN-93. In transgenic mice with targeted inhibition of CaMKII at the SR membranes (SR-AIP), PBs were significantly decreased from 31±6 to 5±1 beats/3min with a virtually complete disappearance of early-afterdepolarizations (EADs). In mice with genetic mutation of the CaMKII phosphorylation site on RyR2 (RyR2-S2814A), PBs decreased by 51.0±14.7%. In contrast, the number of PBs upon reperfusion did not change in transgenic mice with ablation of both PLN phosphorylation sites (PLN-DM). The experiments in SR-AIP mice, in which the CaMKII inhibitor peptide is anchored in the SR membrane but also inhibits CaMKII regulation of L-type Ca(2+) channels, indicated a critical role of CaMKII-dependent phosphorylation of SR proteins and/or L-type Ca(2+) channels in reperfusion arrhythmias. The experiments in RyR2-S2814A further indicate that up to 60% of PBs related to CaMKII are dependent on the phosphorylation of RyR2-Ser2814 site and could be ascribed to delayed-afterdepolarizations (DADs). Moreover, phosphorylation of PLN-Thr17 and L-type Ca(2+) channels might contribute to reperfusion-induced PBs, by increasing SR Ca(2+) content and Ca(2+) influx.
2011 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Ablation of phospholamban rescues reperfusion arrhythmias but exacerbates myocardium infarction in hearts with Ca2+/calmodulin kinase II constitutive phosphorylation of ryanodine receptors.Cardiovasc Res. 2019 Mar 1;115(3):556-569. doi: 10.1093/cvr/cvy213. Cardiovasc Res. 2019. PMID: 30169578 Free PMC article.
-
Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II.Am J Physiol Heart Circ Physiol. 2008 Oct;295(4):H1669-83. doi: 10.1152/ajpheart.00010.2008. Epub 2008 Aug 22. Am J Physiol Heart Circ Physiol. 2008. PMID: 18723772 Free PMC article.
-
Calcium-calmodulin kinase II mediates digitalis-induced arrhythmias.Circ Arrhythm Electrophysiol. 2011 Dec;4(6):947-57. doi: 10.1161/CIRCEP.111.964908. Epub 2011 Oct 18. Circ Arrhythm Electrophysiol. 2011. PMID: 22009705
-
Phospholamban phosphorylation by CaMKII under pathophysiological conditions.Front Biosci. 2008 May 1;13:5988-6005. doi: 10.2741/3131. Front Biosci. 2008. PMID: 18508637 Review.
-
Ryanodine receptor phosphorylation, calcium/calmodulin-dependent protein kinase II, and life-threatening ventricular arrhythmias.Trends Cardiovasc Med. 2011 Feb;21(2):48-51. doi: 10.1016/j.tcm.2012.02.004. Trends Cardiovasc Med. 2011. PMID: 22578240 Free PMC article. Review.
Cited by
-
Arsenic trioxide triggered calcium homeostasis imbalance and induced endoplasmic reticulum stress-mediated apoptosis in adult rat ventricular myocytes.Toxicol Res (Camb). 2016 Feb 8;5(2):682-688. doi: 10.1039/c5tx00463b. eCollection 2016 Mar 1. Toxicol Res (Camb). 2016. PMID: 30090381 Free PMC article.
-
CaMKII-dependent responses to ischemia and reperfusion challenges in the heart.Front Pharmacol. 2014 May 6;5:96. doi: 10.3389/fphar.2014.00096. eCollection 2014. Front Pharmacol. 2014. PMID: 24834054 Free PMC article. Review.
-
Cardiac arrhythmias in patients with COVID-19.J Arrhythm. 2020 Jul 26;36(5):827-836. doi: 10.1002/joa3.12405. eCollection 2020 Oct. J Arrhythm. 2020. PMID: 33024460 Free PMC article. Review.
-
CaMKII-dependent phosphorylation of cardiac ryanodine receptors regulates cell death in cardiac ischemia/reperfusion injury.J Mol Cell Cardiol. 2014 Sep;74:274-83. doi: 10.1016/j.yjmcc.2014.06.004. Epub 2014 Jun 17. J Mol Cell Cardiol. 2014. PMID: 24949568 Free PMC article.
-
The role of CaMKII regulation of phospholamban activity in heart disease.Front Pharmacol. 2014 Jan 27;5:5. doi: 10.3389/fphar.2014.00005. eCollection 2014. Front Pharmacol. 2014. PMID: 24550830 Free PMC article. Review.
References
-
- Manning AS, Coltart DJ, Hearse DJ. Ischemia and reperfusion-induced arrhythmias in the rat. Effects of xanthine oxidase inhibition with allopurinol. Circ Res. 1984;55:545–548. - PubMed
-
- Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. - PubMed
-
- Salerno JA, Previtali M, Chimienti M, Klersy C, Bobba P. Vasospasm and ventricular arrhythmias. NY Acad Sci. 1984;427:222–233. - PubMed
-
- Terkelsen CJ, Sørensen JT, Kaltoft AK, Nielsen SS, Thuesen L, Bøtker HE, et al. Prevalence and significance of accelerated idioventricular rhythm in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2009;104:1641–1646. - PubMed
-
- Pride YB, Appelbaum E, Lord EE, Sloan S, Cannon CP, Sabatine MS, et al. TIMI Study Group. Relation between myocardial infarct size and ventricular tachyarrhythmia among patients with preserved left ventricular ejection fraction following fibrinolytic therapy for ST-segment elevation myocardial infarction. Am J Cardiol. 2009;104:475–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

