Similar endothelial glycocalyx structures in microvessels from a range of mammalian tissues: evidence for a common filtering mechanism?
- PMID: 21889441
- PMCID: PMC3164174
- DOI: 10.1016/j.bpj.2011.07.036
Similar endothelial glycocalyx structures in microvessels from a range of mammalian tissues: evidence for a common filtering mechanism?
Abstract
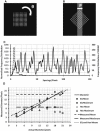
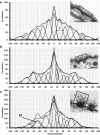
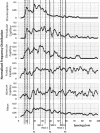
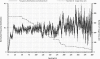
The glycocalyx or endocapillary layer on the luminal surface of microvessels has a major role in the exclusion of macromolecules from the underlying endothelial cells. Current structural evidence in the capillaries of frog mesentery indicates a regularity in the structure of the glycocalyx, with a center-to-center fiber spacing of 20 nm and a fiber width of 12 nm, which might explain the observed macromolecular filtering properties. In this study, we used electron micrographs of tissues prepared using perfusion fixation and tannic acid treatment. The digitized images were analyzed using autocorrelation to find common spacings and to establish whether similar structures, hence mechanisms, are present in the microvessel glycocalyces of a variety of mammalian tissues. Continuous glycocalyx layers in mammalian microvessels of choroid, renal tubules, glomerulus, and psoas muscle all showed similar lateral spacings at ∼19.5 nm (possibly in a quasitetragonal lattice) and longer spacings above 100 nm. Individual glycocalyx tufts above fenestrations in the first three of these tissues and also in stomach fundus and jejunum showed evidence for similar short-range structural regularity, but with more disorder. The fiber diameter was estimated as 18.8 (± 0.2) nm, but we believe this is an overestimate because of the staining method used. The implications of these findings are discussed.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Luft J.H. Fine structure of capillaries: the endocapillary layer. Anat. Record. 1965;151:380.
-
- Levick J.R., Michel C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010;87:198–210. - PubMed
-
- Vink H., Duling B.R. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ. Res. 1996;79:581–589. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

