Structure and dynamics of oligomeric intermediates in β2-microglobulin self-assembly
- PMID: 21889462
- PMCID: PMC3164137
- DOI: 10.1016/j.bpj.2011.07.023
Structure and dynamics of oligomeric intermediates in β2-microglobulin self-assembly
Abstract
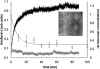
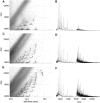
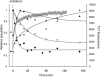
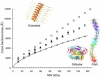
β(2)-Microglobulin is a 99-residue protein with a propensity to form amyloid-like fibrils in vitro which exhibit distinct morphologies dependent on the solution conditions employed. Here we have used ion mobility spectrometry-mass spectrometry to characterize the oligomeric species detected during the formation of worm-like fibrils of β(2)-microglobulin at pH 3.6. Immediately upon sample dissolution, β(2)-microglobulin monomer and oligomers-the latter ranging in size from dimer to hexamer-are present as a pool of rapidly interconverting species. Increasing the ionic strength of the solution initiates fibril formation without a lag-phase whereupon these oligomers become more stable and higher-order species (7-mer to >14-mer) are observed. The oligomers detected have collision cross-sectional areas consistent with a linearly stacked assembly comprising subunits of native-like volume. The results provide insights into the identity and properties of the transient, oligomeric intermediates formed during assembly of worm-like fibrils and identify species that differ significantly from the oligomers previously characterized during the nucleated assembly of long, straight fibrils. The data presented demonstrate the interrelationship between different fibril-forming pathways and identify their points of divergence.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Elongated oligomers in beta2-microglobulin amyloid assembly revealed by ion mobility spectrometry-mass spectrometry.Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):6794-8. doi: 10.1073/pnas.0913046107. Epub 2010 Mar 29. Proc Natl Acad Sci U S A. 2010. PMID: 20351246 Free PMC article.
-
Investigating the structural properties of amyloid-like fibrils formed in vitro from beta2-microglobulin using limited proteolysis and electrospray ionisation mass spectrometry.Rapid Commun Mass Spectrom. 2006;20(11):1628-36. doi: 10.1002/rcm.2482. Rapid Commun Mass Spectrom. 2006. PMID: 16636995 Free PMC article.
-
Competing pathways determine fibril morphology in the self-assembly of beta2-microglobulin into amyloid.J Mol Biol. 2005 Aug 26;351(4):850-64. doi: 10.1016/j.jmb.2005.06.040. J Mol Biol. 2005. PMID: 16024039
-
Limited proteolysis in the investigation of beta2-microglobulin amyloidogenic and fibrillar states.Biochim Biophys Acta. 2005 Nov 10;1753(1):44-50. doi: 10.1016/j.bbapap.2005.09.004. Epub 2005 Sep 23. Biochim Biophys Acta. 2005. PMID: 16213198 Review.
-
Glimpses of the molecular mechanisms of beta2-microglobulin fibril formation in vitro: aggregation on a complex energy landscape.FEBS Lett. 2009 Aug 20;583(16):2623-9. doi: 10.1016/j.febslet.2009.05.005. Epub 2009 May 9. FEBS Lett. 2009. PMID: 19433089 Free PMC article. Review.
Cited by
-
Integrating mass spectrometry of intact protein complexes into structural proteomics.Proteomics. 2012 May;12(10):1547-64. doi: 10.1002/pmic.201100520. Proteomics. 2012. PMID: 22611037 Free PMC article. Review.
-
Robotically assisted titration coupled to ion mobility-mass spectrometry reveals the interface structures and analysis parameters critical for multiprotein topology mapping.Anal Chem. 2013 Dec 3;85(23):11360-8. doi: 10.1021/ac402276k. Epub 2013 Nov 11. Anal Chem. 2013. PMID: 24164205 Free PMC article.
-
The Early Phase of β2-Microglobulin Aggregation: Perspectives From Molecular Simulations.Front Mol Biosci. 2020 Sep 29;7:578433. doi: 10.3389/fmolb.2020.578433. eCollection 2020. Front Mol Biosci. 2020. PMID: 33134317 Free PMC article. Review.
-
Visualizing and trapping transient oligomers in amyloid assembly pathways.Biophys Chem. 2021 Jan;268:106505. doi: 10.1016/j.bpc.2020.106505. Epub 2020 Nov 10. Biophys Chem. 2021. PMID: 33220582 Free PMC article. Review.
-
Structural Heterogeneity in the Preamyloid Oligomers of β-2-Microglobulin.J Mol Biol. 2020 Jan 17;432(2):396-409. doi: 10.1016/j.jmb.2019.10.030. Epub 2019 Nov 9. J Mol Biol. 2020. PMID: 31711963 Free PMC article.
References
-
- Heegaard N.H. β2-microglobulin: from physiology to amyloidosis. Amyloid. 2009;16:151–173. - PubMed
-
- Gosal W.S., Morten I.J., Radford S.E. Competing pathways determine fibril morphology in the self-assembly of β2-microglobulin into amyloid. J. Mol. Biol. 2005;351:850–864. - PubMed
-
- Smith A.M., Jahn T.R., Radford S.E. Direct observation of oligomeric species formed in the early stages of amyloid fibril formation using electrospray ionization mass spectrometry. J. Mol. Biol. 2006;364:9–19. - PubMed
-
- Myers S.L., Thomson N.H., Ashcroft A.E. Investigating the structural properties of amyloid-like fibrils formed in vitro from β2-microglobulin using limited proteolysis and electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20:1628–1636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

