Myocardial perfusion and contraction in acute ischemia and chronic ischemic heart disease
- PMID: 21889943
- PMCID: PMC3251640
- DOI: 10.1016/j.yjmcc.2011.08.019
Myocardial perfusion and contraction in acute ischemia and chronic ischemic heart disease
Abstract
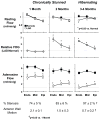
A large body of evidence has demonstrated that there is a close coupling between regional myocardial perfusion and contractile function. When ischemia is mild, this can result in the development of a new balance between supply and energy utilization that allows the heart to adapt for a period of hours over which myocardial viability can be maintained, a phenomenon known as "short-term hibernation". Upon reperfusion after reversible ischemia, regional myocardial function remains depressed. The "stunned myocardium" recovers spontaneously over a period of hours to days. The situation in myocardium subjected to chronic repetitive ischemia is more complex. Chronic dysfunction can initially reflect repetitive stunning with insufficient time for the heart to recover between episodes of spontaneous ischemia. As the frequency and/or severity of ischemia increases, the heart undergoes a series of adaptations which downregulate metabolism to maintain myocyte viability at the expense of contractile function. The resulting "hibernating myocardium" develops regional myocyte cellular hypertrophy as a compensatory response to ischemia-induced apoptosis along with a series of molecular adaptations that while regional, are similar to global changes found in advanced heart failure. As a result, flow-function relations become independently affected by tissue remodeling and interventions that stimulate myocyte regeneration. Similarly, chronic vascular remodeling may alter flow regulation in a fashion that increases myocardial vulnerability to ischemia. Here we review our current understanding of myocardial flow-function relations during acute ischemia in normal myocardium and highlight newly identified complexities in their interpretation in viable chronically dysfunctional myocardium with myocyte cellular and molecular remodeling. This article is part of a Special Issue entitled "Coronary Blood Flow".
Published by Elsevier Ltd.
Figures
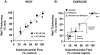








References
-
- Canty JM., Jr . Coronary blood flow and myocardial ischemia. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease. 9. Philadelphia: Elsevier; 2012. pp. 1049–75.
-
- Ross J., Jr Myocardial perfusion-contraction matching: Implications for coronary heart disease and hibernation. Circulation. 1991;83:1076–83. - PubMed
-
- Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–9. - PubMed
-
- Bolli R. Myocardial ‘stunning’ in man. Circulation. 1992;86:1671–91. - PubMed
-
- Braunwald E, Kloner RA. The stunned myocardium: Prolonged, postischemic ventricular dysfunction. Circulation. 1982;66:1146–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

