Activation of the mTOR pathway by low levels of xenoestrogens in breast epithelial cells from high-risk women
- PMID: 21890461
- PMCID: PMC3204351
- DOI: 10.1093/carcin/bgr196
Activation of the mTOR pathway by low levels of xenoestrogens in breast epithelial cells from high-risk women
Abstract
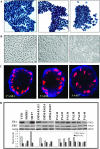
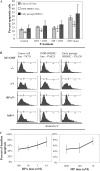
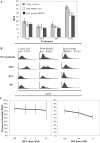
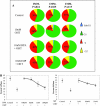
Breast cancer is an estrogen-driven disease. Consequently, hormone replacement therapy correlates with disease incidence. However, increasing male breast cancer rates over the past three decades implicate additional sources of estrogenic exposure including wide spread estrogen-mimicking chemicals or xenoestrogens (XEs), such as bisphenol-A (BPA). By exposing renewable, human, high-risk donor breast epithelial cells (HRBECs) to BPA at concentrations that are detectable in human blood, placenta and milk, we previously identified gene expression profile changes associated with activation of mammalian target of rapamycin (mTOR) pathway genesets likely to trigger prosurvival changes in human breast cells. We now provide functional validation of mTOR activation using pairwise comparisons of 16 independent HRBEC samples with and without BPA exposure. We demonstrate induction of key genes and proteins in the PI3K-mTOR pathway--AKT1, RPS6 and 4EBP1 and a concurrent reduction in the tumor suppressor, phosphatase and tensin homolog gene protein. Altered regulation of mTOR pathway proteins in BPA-treated HRBECs led to marked resistance to rapamycin, the defining mTOR inhibitor. Moreover, HRBECs pretreated with BPA, or the XE, methylparaben (MP), surmounted antiestrogenic effects of tamoxifen showing dose-dependent apoptosis evasion and induction of cell cycling. Overall, XEs, when tested in benign breast cells from multiple human subjects, consistently initiated specific functional changes of the kind that are attributed to malignant onset in breast tissue. Our observations demonstrate the feasibility of studying renewable human samples as surrogates and reinforce the concern that BPA and MP, at low concentrations detected in humans, can have adverse health consequences.
Figures

References
-
- Darbre PD, et al. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human risks. J. Appl. Toxicol. 2008;28:561–578. - PubMed
-
- Statistics Canada. Bisphenol A concentrations in the Canadian population, 2007 to 2009. http://www.statcan.gc.ca/pub/82-003-x/2010003/article/11324/tbl/tbl1-eng... (17 April 2011, date last accessed)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

