Common variants of the BRCA1 wild-type allele modify the risk of breast cancer in BRCA1 mutation carriers
- PMID: 21890493
- PMCID: PMC3733139
- DOI: 10.1093/hmg/ddr388
Common variants of the BRCA1 wild-type allele modify the risk of breast cancer in BRCA1 mutation carriers
Abstract
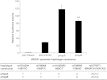
Mutations in the BRCA1 gene substantially increase a woman's lifetime risk of breast cancer. However, there is great variation in this increase in risk with several genetic and non-genetic modifiers identified. The BRCA1 protein plays a central role in DNA repair, a mechanism that is particularly instrumental in safeguarding cells against tumorigenesis. We hypothesized that polymorphisms that alter the expression and/or function of BRCA1 carried on the wild-type (non-mutated) copy of the BRCA1 gene would modify the risk of breast cancer in carriers of BRCA1 mutations. A total of 9874 BRCA1 mutation carriers were available in the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) for haplotype analyses of BRCA1. Women carrying the rare allele of single nucleotide polymorphism rs16942 on the wild-type copy of BRCA1 were at decreased risk of breast cancer (hazard ratio 0.86, 95% confidence interval 0.77-0.95, P = 0.003). Promoter in vitro assays of the major BRCA1 haplotypes showed that common polymorphisms in the regulatory region alter its activity and that this effect may be attributed to the differential binding affinity of nuclear proteins. In conclusion, variants on the wild-type copy of BRCA1 modify risk of breast cancer among carriers of BRCA1 mutations, possibly by altering the efficiency of BRCA1 transcription.
Figures



References
-
- Antoniou A., Pharoah P.D., Narod S., Risch H.A., Eyfjord J.E., Hopper J.L., Loman N., Olsson H., Johannsson O., Borg A., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am. J. Hum. Genet. 2003;72:1117–1130. doi:10.1086/375033. - DOI - PMC - PubMed
-
- Antoniou A.C., Cunningham A.P., Peto J., Evans D.G., Lalloo F., Narod S.A., Risch H.A., Eyfjord J.E., Hopper J.L., Southey M.C., et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br. J. Cancer. 2008;98:1457–1466. doi:10.1038/sj.bjc.6604305. - DOI - PMC - PubMed
-
- Ford D., Easton D.F., Bishop D.T., Narod S.A., Goldgar D.E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692–695. doi:10.1016/S0140-6736(94)91578-4. - DOI - PubMed
-
- Begg C.B., Haile R.W., Borg A., Malone K.E., Concannon P., Thomas D.C., Langholz B., Bernstein L., Olsen J.H., Lynch C.F., et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA. 2008;299:194–201. doi:10.1001/jama.2007.55-a. - DOI - PMC - PubMed
-
- Simchoni S., Friedman E., Kaufman B., Gershoni-Baruch R., Orr-Urtreger A., Kedar-Barnes I., Shiri-Sverdlov R., Dagan E., Tsabari S., Shohat M., et al. Familial clustering of site-specific cancer risks associated with BRCA1 and BRCA2 mutations in the Ashkenazi Jewish population. Proc. Natl Acad. Sci. USA. 2006;103:3770–3774. doi:10.1073/pnas.0511301103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA069417/CA/NCI NIH HHS/United States
- U01 CA069638/CA/NCI NIH HHS/United States
- R01-CA102776/CA/NCI NIH HHS/United States
- U01 CA69417/CA/NCI NIH HHS/United States
- U01 CA069398/CA/NCI NIH HHS/United States
- CA74415/CA/NCI NIH HHS/United States
- R01 CA083855/CA/NCI NIH HHS/United States
- P30 CA051008/CA/NCI NIH HHS/United States
- R01 CA102776/CA/NCI NIH HHS/United States
- U01 CA069446/CA/NCI NIH HHS/United States
- U01 CA69467/CA/NCI NIH HHS/United States
- R01 CA140323/CA/NCI NIH HHS/United States
- R01CA140323/CA/NCI NIH HHS/United States
- CA128978/CA/NCI NIH HHS/United States
- 11174/CRUK_/Cancer Research UK/United Kingdom
- P30-CA051008/CA/NCI NIH HHS/United States
- U01 CA069467/CA/NCI NIH HHS/United States
- RFA-CA-06-503/CA/NCI NIH HHS/United States
- 11022/CRUK_/Cancer Research UK/United Kingdom
- N02-CP-11019-50/CP/NCI NIH HHS/United States
- CAPMC/ CIHR/Canada
- R01 CA128978/CA/NCI NIH HHS/United States
- N02 CP011019/CP/NCI NIH HHS/United States
- N02 CP065504/CP/NCI NIH HHS/United States
- G0801875/MRC_/Medical Research Council/United Kingdom
- U01 CA113916/CA/NCI NIH HHS/United States
- U01 CA069631/CA/NCI NIH HHS/United States
- U01 CA69398/CA/NCI NIH HHS/United States
- R01-CA083855/CA/NCI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- 5U01CA113916/CA/NCI NIH HHS/United States
- U01 CA69638/CA/NCI NIH HHS/United States
- U01 CA69446/CA/NCI NIH HHS/United States
- U01 CA69631/CA/NCI NIH HHS/United States
- U01CA69631/CA/NCI NIH HHS/United States
- R01 CA074415/CA/NCI NIH HHS/United States
- 10118/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

