The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome
- PMID: 21890643
- PMCID: PMC3208300
- DOI: 10.1182/blood-2011-07-368084
The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome
Abstract
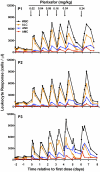
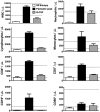
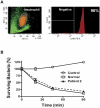
WHIM syndrome is a rare congenital immunodeficiency disorder characterized by warts, hypogammaglobulinemia, infections, and myelokathexis (neutropenia because of impaired egress from the BM); most patients also have severe panleukopenia. Because WHIM syndrome is caused by mutations in the chemokine receptor CXCR4 that result in increased agonist-dependent signaling, we hypothesized that the CXCR4 antagonist plerixafor (Mozobil [Genyzme Corporation], AMD3100), might be an effective treatment. To test this, we enrolled 3 unrelated adult patients with the most common WHIM mutation, CXCR4(R334X), in a phase 1 dose-escalation study. Plerixafor increased absolute lymphocyte, monocyte, and neutrophil counts in blood to normal without significant side effects in all 3 patients. Peak responses occurred at 3-12 hours after injection and waned by 24 hours after injection which tracked the drug's pharmacokinetics. All 3 cell types increased in a dose-dependent manner with the rank order of responsiveness absolute lymphocyte > monocyte > neutrophil. These data provide the first pharmacologic evidence that panleukopenia in WHIM syndrome is caused by CXCL12-CXCR4 signaling-dependent leukocyte sequestration, and support continued study of plerixafor as mechanism-based therapy in this disease. This study is registered at http://www.clinicaltrials.gov as NCT00967785.
Figures


Comment in
-
Released on a WHIM.Blood. 2011 Nov 3;118(18):4764-5. doi: 10.1182/blood-2011-08-375162. Blood. 2011. PMID: 22053172 No abstract available.
References
-
- Zuelzer WW. “Myelokathexis”—a new form of chronic granulocytopenia. Report of a case. N Engl J Med. 1964;270:699–704. - PubMed
-
- Krill CE, Jr, Smith HD, Mauer AM. Chronic idiopathic granulocytopenia. N Engl J Med. 1964;270:973–979. - PubMed
-
- Wetzler M, Talpaz M, Kleinerman ES, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89(5):663–672. - PubMed
-
- Hernandez PA, Gorlin RJ, Lukens JN, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34(1):70–74. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

