Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping
- PMID: 21890688
- PMCID: PMC3213974
- DOI: 10.1152/ajpheart.00260.2011
Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping
Abstract
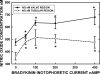
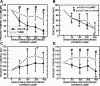
Microscopic lymphatics produce nitric oxide (NO) during contraction as flow shear activates the endothelial cells. The valve leaflets and bulbous valve housing contain a large amount of endothelial nitric oxide synthase (eNOS) due both to many endothelial cells and increased expression of eNOS. Direct NO measurements indicate the valve area has a 30-50% higher NO concentration ([NO]) than tubular regions although both regions generate equivalent relative increases in [NO] with each contraction. We hypothesize that 1) the greater eNOS and [NO] of the bulb region would have greater effects to lower pumping activity of the overall lymphatic than occurs in tubular regions and 2), the elevated [NO] in the bulb region may be because of high NO production in the valve leaflets that diffuses to the wall of the bulb. Measurement of [NO] with a micropipette inside the lymphatic bulb revealed the valve leaflets generate ~50% larger [NO] than the bulb wall in the in vivo rat mesenteric lymphatics. The valves add NO to the lymph that quickly diffuses to the bulb wall. Bradykinin locally released iontophoretically from a micropipette on both bulbs and tubes increased the [NO] in a dose-dependent manner up to ~50%, demonstrating agonist activation of the NO pathway. However, pumping output determined by contraction frequency and stroke volume decreased much more for the bulb than tubular areas in response to the bradykinin. In effect, NO generation by the bulb area and its valves limits the pumped flow of the total lymphatic by lowering frequency and stroke volume of individual contractions.
Figures




References
-
- Bauser-Heaton HD, Bohlen HG. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS 2. Am J Physiol Heart Circ Physiol 293: H2193–H2201, 2007 - PubMed
-
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 - PubMed
-
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress 7. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 - PubMed
-
- Bohlen HG. Intestinal mucosal oxygenation influences absorptive hyperemia. Am J Physiol Heart Circ Physiol 239: H489–H493, 1980 - PubMed
-
- Bohlen HG. Integration of intestinal structure, function, and microvascular regulation. Microcirculation 5: 27–37, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

